Abstract
There is strong converging evidence that the intermediate and medial part of the hyperstriatum ventrale of the chick brain is a memory store for information acquired through the learning process of imprinting. Neurons in this memory system come, through imprinting, to respond selectively to the imprinting stimulus (IS) neurons and so possess the properties of a memory trace. Therefore, the responses of the intermediate and medial part of the hyperstriatum ventrale neurons to a visual imprinting stimulus were determined before, during, and after training. Of the total recorded population, the proportions of IS neurons shortly after each of two 1-h training sessions were significantly higher (approximately 2 times) than the pretraining proportion. However, ≈4.5 h later this proportion had fallen significantly and did not differ significantly from the pretraining proportion. Nevertheless, ≈21.5 h after the end of training, the proportion of IS neurons was at its highest (approximately 3 times the pretraining level). No significant fluctuations occurred in the proportions of neurons responding to the alternative stimulus. In addition, nonmonotonic changes were found commonly in the activity of 230 of the neurons tracked individually from before training to shortly after the end of training. Thus the pattern of change in responsiveness both at the population level and at the level of individual neurons was highly nonmonotonic. Such a pattern of change is not consistent with simple models of memory based on synaptic strengthening to asymptote. A model is proposed that accounts for the changes in the population responses to the imprinting stimulus in terms of changes in the responses of individual neurons.
A particular stimulus or event that is committed to memory is widely held to be represented in the brain as a neural trace, or “engram” (1–4). Despite the fact that more than 50 years have elapsed since Lashley published his landmark paper entitled “In search of the engram” (4), the nature of the trace has proved elusive. A major impediment to advance has been the difficulty of identifying brain regions in which memory traces are known to be formed (5). This difficulty has largely been overcome in the case of visual imprinting in domestic chicks when the young birds learn the characteristics of and preferentially approach an object to which they have been exposed (6). Because this approach is selective for the familiar stimulus it is supposed that a neural representation of this stimulus is formed, as in other instances of recognition memory (3).
Strong converging evidence indicates that a restricted part of the forebrain stores this representation (7, 8). Shortly after chicks have been trained by exposing them to an imprinting stimulus (training), there is an increase in the incorporation of [3H]uracil into RNA in the dorsal (“roof”), but not the ventral part of the cerebral hemispheres (9). The regional increase is closely related to learning because (i) when visual input was restricted to one cerebral hemisphere incorporation was higher in the roof of the “trained” than the “untrained” hemisphere (10); (ii) the magnitude of the increase was positively correlated with a measure of how much the chicks had learned (11), and (iii) the increase could not be attributed to short-lasting effects of sensory stimulation (12). By using autoradiographic techniques, a training-related increase in the incorporation of radioactive uracil into RNA was found in the intermediate and medial part of the hyperstriatum ventrale (IMHV) but not in any of the other forebrain regions sampled (13). Imprinting leads to an increase in length of the postsynaptic densities of excitatory (axospinous) synapses in this region but not in a visual projection area, the hyperstriatum accessorium (14, 15). Particular changes occurring in IMHV after training are positively correlated with the amount chicks learn and cannot be attributed to side-effects of the training, such as sensory stimulation, motor activity, or arousal. These changes include inter alia (7, 8), an increase in the number of neurons immunopositive for the immediate early gene product Fos (16), an up-regulation of N-methyl-d-aspartate receptors (17, 18), increases in the amounts of neural cell adhesion molecule proteins (19, 20), and an increase in the amount of clathrin (21), a protein that is involved in the recycling of synaptic vesicles (22). Furthermore, experiments involving lesions of both left and right IMHV together or sequentially, before or after imprinting establish that this region, but not certain other regions studied, is necessary for the acquisition and retention of an imprinted preference. These experiments also disclosed the existence of a storage system, S′, that is outside IMHV and operates in parallel with it; S′ becomes functional between 6 and 8 h after the start of training (23–27).
The trace that is formed through learning is thought to represent and to be activated by the learned stimulus (cf. ref. 3, p. 72). Imprinting leads to a substantial increase in the proportion of neurons in IMHV, but not in the hippocampus, that respond to the imprinted stimulus (28–30). Some of these IMHV neurons respond to the imprinted stimulus in a highly selective way and so possess the postulated properties of the memory trace (3). In the present study, we have followed the development of the trace by tracking the responses of IMHV neurons before, during, and after training.
Materials and Methods
Domestic chicks (Gallus gallus domesticus) were used, 14 were trained and 7 were not. Approximately 10 h after hatching, a microelectrode assembly was attached to the skull of each chick under general anesthesia (28, 29). The assembly allowed four microelectrodes to be lowered into IMHV, two into the left and two into the right. In each hemisphere, the electrode tips were separated by ≈200 μm, one anterior to the other. The day after the operation a chick was transferred to a running wheel (Fig. 1) in a dimly illuminated cabinet, and the electrodes were advanced until spontaneous impulse activity was recorded. Neuronal responsiveness to each of two rotating, internally illuminated stimuli, a red box (RB) and a blue cylinder (BC), presented in sequence was then measured [“Test”, T1 (mean mid-point of recording period ≈0.75 h before the onset of training), see Fig. 3A]. Approximately 15 min after the end of T1, chicks that were to be trained were exposed for 1 h to the imprinting stimulus, either the BC (seven chicks) or RB (seven chicks). Each stimulus rotated 30 times/min (28, 29). Fifteen minutes after this period of training, the second Test, T2, was given (mean mid-point of recording period ≈1.75 h after the onset of training). After an ≈15-min rest period, the chick was given a second 1-h period of training. After a further ≈15-min rest, the third Test, T3, was given (≈4.25 h after training onset). Chicks remained in the running wheel throughout the period spanning T1–T3. The timing of Test T4 was chosen to be at a time when S′ is known to have formed (27), ≈8 h after the start of training. The fifth Test, T5, was given ≈25 h (mean mid-point of recording period) after the start of training because this corresponded to the time at which tests of neuronal responsiveness commenced in two previous studies (28, 29). Tests of neuronal responsiveness lasted for ≈1 h. Rotations of the running wheel caused by the chick's approach activity toward a stimulus were measured during Tests T1–T5. Two hours after the end of the second training session, the chicks were given a preference test (23). In this test the approach of each trained chick to the imprinting stimulus and to the alternative stimulus, respectively, were determined and a preference score calculated. A score of 50% indicates that a chick approached the stimuli equally in the test (23). The trained chicks preferred the imprinting stimulus: the mean (±SEM.) preference score was 64.9 ± 6.6%, significantly above 50% (t13 = 2.3, P < 0.05). After T3 chicks remained in individual compartments in a dark incubator at all times, except during preference or neuronal responsiveness testing. Recordings from T1 through T3 were made at the same site, and the activity of individual neurons was followed throughout these Tests. Because it was not possible reliably to record from individual neurons in the behaving chick for >7 h (beyond T3), and to increase the size of the sample of recorded neurons, the electrodes were advanced by ≈115 μm ≈30 min before T4 and T5. Action potentials that crossed either a positive or a negative “waveform detection” threshold set at approximately four times the background noise level (31) were sampled at 12.5 kHz, digitized, and recorded for off-line analysis. Action potentials from simultaneously recorded individual neurons (1–12 per electrode) were software sorted (spike2, Cambridge Electronic Design, Cambridge, England) and reliably discriminated by their waveforms (31–33); see for example waveforms 1 and 2 in Fig. 4H. For untrained chicks, neuronal responsiveness was tested at times corresponding to those for trained chicks. Neuronal responsiveness was determined by presenting a stimulus for 4 s (two complete rotations) ≈15–25 times at intervals of ≈15–60 s (28, 29). The order of presenting these series of stimuli was varied across Tests in a quasi-random fashion. The stimulus was presented only when the chick in the running wheel was looking in the direction of the stimulus (28, 29). A neuron was defined as responsive to a stimulus if its mean firing rate during presentations of the stimulus differed significantly from that in the 4 s preceding the presentations (two-tailed paired t test, P < 0.05). Some neurons (≈25%) in the previous studies (ref. 28 and Table 3 of ref. 29) responded to the imprinting stimulus and were classified as such even if these neurons did not discriminate between it and the nonimprinting stimulus. For the purposes of comparison with these studies (Fig. 2C), we adopted the same method of classification. Otherwise we have concentrated on those neurons [imprinting stimulus (IS) neurons] that specifically signaled the presence of the imprinting stimulus by meeting two criteria: (i) responding significantly to this stimulus and (ii) not responding significantly to the nonimprinting stimulus. Neurons that responded to a stimulus not seen in training (to the alternative stimulus in trained chicks or to either of the BC or RB in untrained chicks) are referred to as nonimprinting stimulus, NIS, neurons. The proportions of neurons responsive to each stimulus in each Test were analyzed by using ANOVAs based on a generalized linear model assuming a binomial error distribution (34). The ANOVAs incorporated the factors neuron type (IS, NIS) and test (T1–T5). Additional factors, which were without significant effect on the results reported, were chick, hemisphere (left or right), position of electrode in hemisphere (anterior or posterior), and two factors indicating whether the activity of a neuron was related to approach activity (31). Significant results from these ANOVAs are identified in the text as binomial error model (BEM) χ2 values.
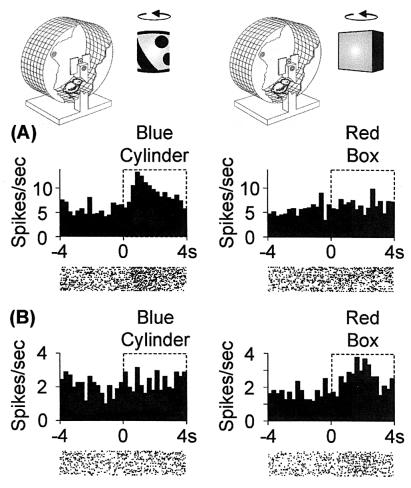
Figure 1.
Responses of IMHV neurons. Above the upper histogram bars are shown chicks in running wheels. For purposes of illustration one of the opaque sides of each wheel is cut away. Chicks face a rotating BC on the left and a rotating RB on the right. (A and B) Example response histograms and raster plots of responses to either the RB or the BC. Neurons were tested for responsiveness to each stimulus. (A) Neuron in BC-trained chick responding to the BC but not RB. (B) Neuron in an RB-trained chick responding to the RB but not BC.
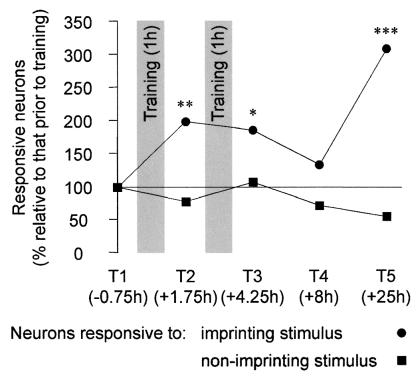
Figure 3.
Population neuronal responsiveness to the imprinting and nonimprinting
stimuli. The change (%) in the proportions of neurons responsive to
the IS (●) and a NIS (■), relative to the
proportion responsive before training are shown for Tests T1–T5 (the
mean mid-points of the Tests relative to the start of training are
indicated). The proportion of neurons responsive to the IS increased
significantly to T2 (BEMχ = 6.7,
**, P < 0.01) and T3 (BEM
χ
= 6.7,
**, P < 0.01) and T3 (BEM
χ = 5.2, *,
P = 0.023). At T4 the proportion of neurons
responsive to the IS was significantly less than the pooled proportions
at T2 and T3 (BEMχ
= 5.2, *,
P = 0.023). At T4 the proportion of neurons
responsive to the IS was significantly less than the pooled proportions
at T2 and T3 (BEMχ = 4.5,
P = 0.034) and was not significantly different from
that at T1. At T5 the proportion of neurons responsive to the IS was
significantly higher than that at T1 (BEM
χ
= 4.5,
P = 0.034) and was not significantly different from
that at T1. At T5 the proportion of neurons responsive to the IS was
significantly higher than that at T1 (BEM
χ = 20.8, ***,
P < 0.001) and that at T4 (BEMχ
= 20.8, ***,
P < 0.001) and that at T4 (BEMχ = 4.9,
P = 0.027).
= 4.9,
P = 0.027).
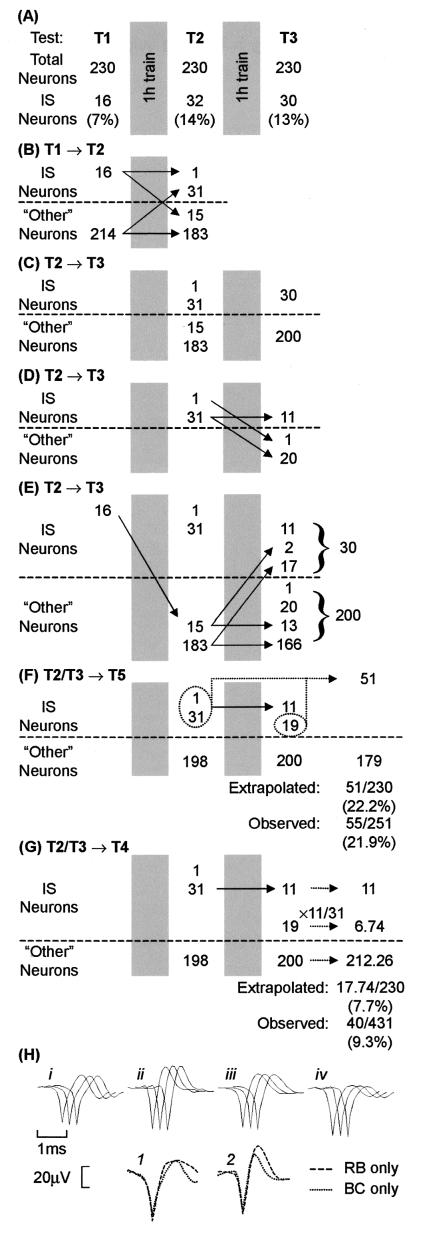
Figure 4.
Tracking the responsiveness of individual neurons. (A) In trained chicks, the activity of 230 neurons was recorded throughout the period spanning T1–T3 and the numbers of neurons responding to the imprinting stimulus (IS neurons) are shown. The pattern of change in neurons responding to the imprinting stimulus, or not doing so (other neurons) are shown for T1→T2 (B) and T2→T3 (C–E). The extrapolated numbers of IS neurons at T5 and T4 are indicated by broken lines in F and G, respectively. The observed and extrapolated proportions of IS neurons in the population are also shown. (H) Averaged action potential waveforms for four neurons are shown.(i–iv) In each set of three waveforms (e.g., i), each waveform (left to right) is reconstructed from activity in Tests T1, T2, and T3, respectively. Each neuron was sampled from a different chick. Neurons responded as follows: (i) IS neuron at T1 and T2, and other at T3, (ii) other at T1, IS at T2, and other at T3; (iii) other at T1 and T2, and IS at T3; and (iv) IS at T1, other at T2, and IS at T3. The averaged action potential waveforms superimposed in 1 have been reconstructed from activity simultaneously sampled through a single microelectrode. Those in 2 were also simultaneously sampled through a single microelectrode, although from a different chick. In both 1 and 2, the two neurons were differentially responsive, one (dashed line) responding to the RB but not to the BC, and the other (dotted line) responding to the BC but not to the RB.
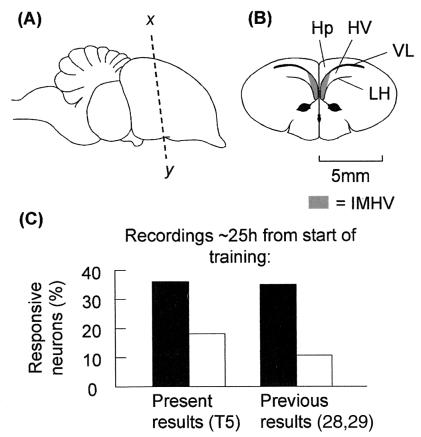
Figure 2.
Proportions of IMHV neurons responding to an imprinting stimulus and a
nonimprinting stimulus. (A) Side view of chick brain.
(B) Frontal section through x–y in
A. The shaded areas show the IMHV region, from which the
recordings were made. Abbreviations: Hp, hippocampus; HV, hyperstriatum
ventrale; LH, lamina hyperstriatica; VL, lateral ventricle.
(C) Proportions of neurons responsive to the imprinting
stimulus (filled bars) and a nonimprinting stimulus (open bars) in the
present (T5) and previous studies (28, 29). The proportions of neurons
responsive to a nonimprinting stimulus in trained and untrained chicks
did not differ significantly and were combined. The proportions of
neurons responsive to the imprinting stimulus were significantly higher
than the proportions responsive to a nonimprinting stimulus (present
results, BEM
χ = 15.6,
P < 0.001; previous results, BEM
χ
= 15.6,
P < 0.001; previous results, BEM
χ = 39.0,
P < 0.001).
= 39.0,
P < 0.001).
Results
Examples of IMHV neuronal responses are shown in Fig. 1. The pattern of responsiveness observed in the present study the day after training (at T5) is similar to that of two previous studies (28, 29) in which recordings were also made the day after training (Fig. 2C, data for left and right IMHV combined).
Population Responses.
In the present study, the proportions of neurons responsive to the
imprinting stimulus (IS neurons), expressed as a percentage of those
responding before training, were determined for each neuronal Test and
are shown in Fig. 3. The proportions of
neurons responsive to the nonimprinting stimulus (NIS neurons) also
were calculated. The proportions of NIS neurons did not vary
significantly across T1–T5. In contrast (IS vs. NIS by Test
interaction: BEM χ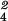 = 19.6,
P < 0.001) the proportions of IS neurons varied
significantly (BEM χ
= 19.6,
P < 0.001) the proportions of IS neurons varied
significantly (BEM χ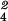 =
15.8, P < 0.01) with these Tests. The
proportion of IS neurons increased significantly after the first period
of training (at T2), and the increase was maintained after the second
period of training (at T3). However, by T4 the proportion of neurons
responsive to the imprinting stimulus had fallen significantly below
the pooled proportions at T2 and T3 and was no longer significantly
above the pretraining level. When tested at T5, there was a highly
significant elevation in the proportion of neurons responsive to the
imprinting stimulus relative to both that at T1 and to that at T4 (see
Fig. 3 and legend).
=
15.8, P < 0.01) with these Tests. The
proportion of IS neurons increased significantly after the first period
of training (at T2), and the increase was maintained after the second
period of training (at T3). However, by T4 the proportion of neurons
responsive to the imprinting stimulus had fallen significantly below
the pooled proportions at T2 and T3 and was no longer significantly
above the pretraining level. When tested at T5, there was a highly
significant elevation in the proportion of neurons responsive to the
imprinting stimulus relative to both that at T1 and to that at T4 (see
Fig. 3 and legend).
Artifactual generation of these nonlinear changes can be ruled out. The recording microelectrodes remained at the same sites in tests T1–T3 but were advanced before T4 and T5. However, movement of the microelectrodes cannot account for the differences in the proportions of IS neurons at these Tests because: (i) at Tests T4 and T5, the proportions of IS neurons were at their posttraining minimum and maximum, respectively, and (ii) in previous studies (28, 29), when recordings were made ≈25 h after the start of training the proportions of neurons responding to the imprinting stimulus did not change as the microelectrode was advanced. The recording sites of T4 and T5 were at different depths within IMHV, but in previous studies (28, 29), the proportions of neurons responding to the imprinting stimulus did not vary with the depth of the penetration. Moreover, there was no significant variation across the Tests, and hence sites, in the mean spontaneous firing rates of IS, NIS, or unresponsive neurons. It is possible that the reduction in the proportion of IS neurons at T4 reflects some general change in behavior, such as a reduction in the level of arousal and alertness. Such a change would be expected to be expressed in a change in the chicks' approach to the training stimulus when it was presented during T4. However, there was no discontinuity in the chicks' approach activity in this Test, and approach activity increased linearly from T1 through T5 (linear regression: r = 0.33, F1,57 = 6.8, P = 0.012). Finally, if any of the above factors influenced the frequency with which IS neurons were encountered, these factors would also be expected similarly to influence the proportions of NIS neurons. There was no significant variation in the proportion of NIS neurons across the Tests (Fig. 3).
Responses of Individually Tracked Neurons.
Do the responses of individual neurons to the imprinting stimulus exhibit nonlinear fluctuations during and after learning, similar to those exhibited by the population of IS neurons? Or do the responses of individual neurons increase continuously to a stable asymptote (the “monotonic hypothesis”) as is implied by certain commonly assumed neural models of memory based on long-term potentiation and Hebbian synapses (3, 35–37)? These questions were investigated by following the activity of 230 individual neurons from Tests T1 through T3. The waveform of the action potentials generated by each of these neurons was stable throughout the recordings (see Fig. 4H, i–iv). As the proportions of NIS neurons were stable across these Tests whereas the proportions of IS neurons varied significantly (Fig. 3), changes were sought in responsiveness of individual IS neurons. For simplicity, neurons were divided into two groups: IS neurons that responded to the imprinting stimulus and “other neurons.” The latter group includes neurons that responded to the nonimprinting stimulus as well as unresponsive neurons. The numbers of IS neurons at T1, T2, and T3 are shown in Fig. 4A. The patterns of change of neurons responding to the imprinting stimulus, or not doing so (other neurons) are followed from T1→T2 (Fig. 4B), and T2→T3 (Fig. 4 C–E). In relation to the monotonic hypothesis, aspects of these findings may by summarized in the following way.
Consistent with the monotonic hypothesis, 31 of the neurons that responded to the imprinting stimulus after the first period of training, at T2, had not been responsive to it before training, at T1 (Fig. 4B). However, the hypothesis cannot adequately account for the overall pattern of change because it predicts that: (i) all 32 IS neurons at T2 should continue to respond to the imprinting stimulus at T3, and (ii) all 16 neurons responding to the imprinting stimulus before training should remain responsive to that stimulus at T3. The data (Fig. 4 D and E, respectively) do not support these predictions (approximation from binomial distribution: t = 7.8, P < 0.001 for the comparison of 32 IS neurons at T2 of which only 11/32 remain IS neurons at T3; t = 10.6, P < 0.001 for the comparison of 16 IS neurons at T1 of which only 2/16 remain IS neurons at T3), and (iii) of the 11 neurons that maintained their responsiveness to the imprinting stimulus at T2 and T3 (Fig. 4D), the magnitude of their response at T3 should either be the same as, or greater than at T2. In fact, their responses at T3 were significantly lower overall than at T2 (sign test, P = 0.012). In all three cases, therefore, the monotonic hypothesis is rejected.
Assuming that the fluctuations in percentages of neurons responding to the imprinting stimulus reflect physiological processes, what might these processes be? One possibility is that a population of neurons becomes responsive to this stimulus, then permanently ceases to do so as another population becomes engaged, and so on—but with a dearth of them responding at T4. In this view, the population of neurons responding to the imprinting stimulus at T5 would be different from the population that responded at, e.g., T2. Another possibility is that individual neurons become responsive after training, but their responsiveness wanes and waxes over time. This possibility was investigated by inquiring whether the proportion of neurons that respond to the imprinting stimulus at T5 and T4 could be predicted by making simple assumptions based on the data from the sample population of the 230 neurons tracked from T1 through T3.
To determine the proportion of responses expected at T5 (≈21 h after the end of training), it is assumed that if a neuron responded to the imprinting stimulus after a period of training, i.e., at T2 or T3, it will respond to that stimulus again at T5 (Fig. 4F). With this assumption, the extrapolated proportion of IS neurons, 51/230 (22.2%) is very close to the observed proportion of 55/251 (21.9%) (Fig. 4F). The extrapolated number of IS neurons at T5 (51/230 × 251 = 55.65) closely predicts the observed number (55 neurons). To determine the proportion of IS neurons expected at T4 (≈4.5 h after the end of training), it is assumed that there will be continuity at T4 of the pattern of stability and fluctuation in the responses to the imprinting stimulus observed after training, at T2 and T3 (Fig. 4 D and E). Thus a proportion of neurons responding to the imprinting stimulus at T2 also responded to it at T3. There were 11 such “stable” IS neurons (Fig. 4D), i.e., 11/31 or 0.355 of the 31 recruited IS neurons of T2. Hence, assuming that the same proportion of the 19 neurons recruited to respond to the imprinting stimulus after the second training period (Fig. 4E) also will be stable IS neurons, 0.355 of these 19 neurons, i.e., 6.74 neurons, should respond as IS neurons at T4. The total number of IS neurons at T4 (Fig. 4G) should therefore be 6.74 + 11 = 17.74, giving an expected proportion of 17.74/230 or 7.7%. The observed percentage was 9.3% (100 × 40/431). The predicted number of IS neurons at T4 is 33.24 (= 17.74/230 × 431), whereas the observed number was 40.
A goodness of fit χ2 test comparing
the observed numbers of IS neurons at T4 and T5 to the extrapolated
numbers was not significant, giving no grounds for rejecting the
model's fit (χ = 1.38, 0.2 < P < 0.3).
The close match between observed and predicted values favors the
hypothesis that through imprinting, some neurons come to respond to the
imprinting stimulus, cease to do so and then after several hours
respond once more. During these hours learning-related changes in gene
expression occur in IMHV (13, 17, 21).
= 1.38, 0.2 < P < 0.3).
The close match between observed and predicted values favors the
hypothesis that through imprinting, some neurons come to respond to the
imprinting stimulus, cease to do so and then after several hours
respond once more. During these hours learning-related changes in gene
expression occur in IMHV (13, 17, 21).
Discussion
Previous work has examined the effects of imprinting on the responsiveness of neurons in a region (IMHV) of the chick brain known to be a memory store for the learning process of imprinting. In those studies, exposure to an imprinting stimulus led to a large and highly significant increase in the proportion of IMHV neurons responding to that stimulus the day after training. In the present study, we have confirmed these findings but found that for most IMHV neurons responding to the imprinting stimulus after training, the changes in responsiveness are highly nonmonotonic. Training initially enhances the responsiveness of some IMHV neurons to the imprinting stimulus, the responsiveness of most of them wanes, and, we propose, recovers again after several hours. What might underlie this sequence of changes in responsiveness?
The initial increase in the number of neurons responding to the imprinting stimulus could occur through a strengthening of synapses in the neural pathways mediating the activity evoked by that stimulus: synapses that at T1 were subthreshold for activating IMHV neurons became suprathreshold after the first period of training, at T2 (Fig. 4B, 31 neurons) much as Hebb (3) envisaged. However, only 11 of these neurons remained responsive to the imprinting stimulus after the second training period; the remaining approximately two thirds ceased to respond to the stimulus at this Test (Fig. 4D). What might account for this waning of responsiveness? Of the 32 neurons that responded to the imprinting stimulus at T2, 21 ceased to do so at T3. Of these 21, six came to respond at T3 to the nonimprinting stimulus whereas 15 became unresponsive to both stimuli. If the failure of responsiveness of these 15 neurons were attributable to postsynaptic inhibition of the recorded neurons, the mean prestimulus spontaneous activity (the firing rate in the 4 s before stimulus onset) of all 15 neurons would be expected to be significantly lower at T3 than at T2. This was the case for only one of them. The response failure of the remaining 14 neurons is therefore unlikely to be brought about by postsynaptic inhibition. Other possibilities are desensitization of receptors in the recorded neurons (38, 39) or a reduction in the amount of transmitter released by the presynaptic input (40). Whatever the reason for the failure to respond, such neurons may become unavailable for incorporation into any other memory system that may be established during their period of unresponsiveness. This unresponsiveness may afford a recently acquired trace some protection from disruption by subsequent learning.
We have suggested that neurons which respond to the imprinting stimulus at T2 or T3 come to respond to the stimulus again at T5. The extrapolated percentage of neurons responding to the imprinting stimulus, based on this assumption (22.2%), is very close to that observed (22.9%). What factors might affect the proposed recovery? Neuronal Test T4 ended ≈8.5 h and neuronal Test T5 began ≈24.5 h after the beginning of training. Each chick spent all of the intervening ≈16 h in its own compartment of the warm (34°C), dark incubator. It is likely that, during some or much of this time the chick was asleep; often in other similar experiments when chicks in the incubator have been inspected under dim green light, their heads are flexed and their eyelids closed. A number of studies in mammals, including humans have suggested that, during sleep, the memory of newly learned information is stabilized (41–43) with different phases of sleep being associated with the stabilization of different forms of memory (44, 45). The stabilizing effects are thought to be mediated by fluctuations in the activity of different neuromodulatory systems (e.g., noradrenergic, serotonergic, cholinergic) and in the levels of plasma glucocorticoids (45, 46) that have been implicated in memory consolidation in mammals (47) and birds (see ref. 7, pp. 218–221). Although it is not known whether any of these factors play a role in enhancing IMHV neuronal responsiveness to the imprinting stimulus from T4 to T5, there is evidence that brief exposure to such a stimulus is followed by an increase in the amount of time spent by young chicks in paradoxical sleep (48).
The long rest period between T4 and T5 contrasts with the shorter period of ≈2.75 h between the end of T3 and the start of T4. At the end of T3, each chick was returned to the incubator for ≈45 min after which the chick was given the preference test (see Materials and Methods). This test lasted ≈30 min after which the chick was again returned to the incubator where it remained for ≈1.5 h. It is possible that these disturbances prevented the chick from sleeping long enough for the putative effects of sleep to be exerted on the memory trace. These considerations raise the possibility that: (i) training transiently increases the strength of a specific set of synapses mediating the response to the imprinting stimulus; (ii) these synapses are in some way “labeled” even though their strength attenuates, and (iii) the strength of these synapses is again enhanced several hours after training through the actions of neuromodulatory substances including glucorticoids engaged during sleep.
When chicks' preferences are tested 8–10 h after training they prefer the training stimulus.† At this time, the proportion of neurons in IMHV that respond to the imprinting stimulus has fallen to the pretraining level, suggesting that IMHV neurons do not then provide the signals necessary for the imprinted stimulus to be recognized. By this time, ≈8 h after the start of training, the storage system S′ is functioning. S′ is formed after and in parallel with the store in IMHV (23–27), is able to sustain an imprinted preference in the absence of IMHV, and would be expected to do so when IMHV neurons cannot provide the information necessary for recognition. If the kind of variation in neuronal responsiveness found in IMHV is found in other memory systems, they may well incorporate a store such as S′, out of phase in its formation and in parallel with an equivalent structure to IMHV. The topic of late-developing stores is of considerable current interest (49–51).
We have tracked the changes in neuronal responses that occur with learning in a real memory system both in individual neurons and in population measures of neuronal responsiveness. These changes are surprisingly nonlinear. Our findings present a challenge to current ideas concerning cellular mechanisms of memory, in particular those that assume that, through learning, the strength of synapses and hence the responsiveness of neurons in a memory system increase monotonically to a stable asymptote (3, 35–37).
Acknowledgments
We are grateful to Steve Ellis, Paul Heavens, and Vicky Mills for their invaluable contributions, Prof. Chris Gilligan for statistical advice, and to the Biotechnology and Biological Sciences Research Council for financial support.
Abbreviations
- IMHV
intermediate and medial part of the hyperstriatum ventrale
- IS
imprinting stimulus
- NIS
nonimprinting stimulus
- RB
rotating red box
- BC
rotating blue cylinder
- BEM
binomial error model
Footnotes
† To whom reprint requests should be addressed. E-mail: gh105@cus.cam.ac.uk.
References
- 1.James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- 2.Cajal S R. Histologie du Système Nerveux de l'Homme et des Vertébrés. Paris: Maloine; 1911. [Google Scholar]
- 3.Hebb D O. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 4.Lashley K S. Symp Soc Exp Biol. 1950;4:454–482. [Google Scholar]
- 5.Kim J J, Thompson R F. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 6.Bolhuis J J. Biol Rev. 1991;66:303–345. doi: 10.1111/j.1469-185x.1991.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 7.Horn G. Memory, Imprinting and the Brain. Oxford: Clarendon Press; 1985. [Google Scholar]
- 8.Horn G. Trends Neurosci. 1998;21:300–306. doi: 10.1016/s0166-2236(97)01219-8. [DOI] [PubMed] [Google Scholar]
- 9.Bateson P P G, Horn G, Rose S P R. Brain Res. 1972;39:449–465. doi: 10.1016/0006-8993(72)90448-9. [DOI] [PubMed] [Google Scholar]
- 10.Horn G, Rose S P R, Bateson P P G. Brain Res. 1973;56:227–237. doi: 10.1016/0006-8993(73)90337-5. [DOI] [PubMed] [Google Scholar]
- 11.Bateson P P G, Horn G, Rose S P R. Brain Res. 1975;84:207–220. doi: 10.1016/0006-8993(75)90976-2. [DOI] [PubMed] [Google Scholar]
- 12.Bateson P P G, Rose S P R, Horn G. Science. 1973;181:576–578. doi: 10.1126/science.181.4099.576. [DOI] [PubMed] [Google Scholar]
- 13.Horn G, McCabe B J, Bateson P P G. Brain Res. 1979;168:361–373. doi: 10.1016/0006-8993(79)90176-8. [DOI] [PubMed] [Google Scholar]
- 14.Bradley P, Horn G, Bateson P. Exp Brain Res. 1981;41:115–120. doi: 10.1007/BF00236600. [DOI] [PubMed] [Google Scholar]
- 15.Horn G, Bradley P, McCabe B J. J Neurosci. 1985;5:3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCabe B J, Horn G. Proc Natl Acad Sci USA. 1994;91:11417–11421. doi: 10.1073/pnas.91.24.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe B J, Horn G. Proc Natl Acad Sci USA. 1988;85:2849–2853. doi: 10.1073/pnas.85.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe B J, Horn G. Behav Neurosci. 1991;105:289–294. doi: 10.1037//0735-7044.105.2.289. [DOI] [PubMed] [Google Scholar]
- 19.Solomonia R O, McCabe B J, Horn G. Behav Neurosci. 1998;112:646–655. doi: 10.1037//0735-7044.112.3.646. [DOI] [PubMed] [Google Scholar]
- 20.Solomonia R O, Kiguradze T, McCabe B J, Horn G. NeuroReport. 2000;11:3139–3143. doi: 10.1097/00001756-200009280-00020. [DOI] [PubMed] [Google Scholar]
- 21.Solomonia R O, McCabe B J, Jackson A P, Horn G. Neuroscience. 1997;80:59–67. doi: 10.1016/s0306-4522(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 22.Morris S A, Schmid S. Curr Biol. 1995;5:113–115. doi: 10.1016/s0960-9822(95)00028-5. [DOI] [PubMed] [Google Scholar]
- 23.McCabe B J, Horn G, Bateson P P G. Brain Res. 1981;205:29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- 24.McCabe B J, Cipolla-Neto J, Horn G, Bateson P. Exp Brain Res. 1982;48:13–21. doi: 10.1007/BF00239568. [DOI] [PubMed] [Google Scholar]
- 25.Cipolla-Neto J, Horn G, McCabe B J. Exp Brain Res. 1982;48:22–27. doi: 10.1007/BF00239569. [DOI] [PubMed] [Google Scholar]
- 26.Horn G, McCabe B J, Cipolla-Neto J. Exp Brain Res. 1983;53:91–98. doi: 10.1007/BF00239401. [DOI] [PubMed] [Google Scholar]
- 27.Honey R C, Horn G, Bateson P, Walpole M. Behav Neurosci. 1995;109:689–698. doi: 10.1037//0735-7044.109.4.689. [DOI] [PubMed] [Google Scholar]
- 28.Brown M W, Horn G. Eur J Neurosci. 1994;6:1479–1490. doi: 10.1111/j.1460-9568.1994.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 29.Nicol A U, Brown M W, Horn G. Eur J Neurosci. 1995;7:766–776. doi: 10.1111/j.1460-9568.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 30.Nicol A U, Brown M W, Horn G. Eur J Neurosci. 1998;10:2738–2741. doi: 10.1046/j.1460-9568.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- 31.Nicol A U, Brown M W, Horn G. Eur J Neurosci. 1998;10:34–44. doi: 10.1046/j.1460-9568.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- 32.McNaughton T G, Horch K W. IEEE Trans Biomed Eng. 1994;41:609–616. doi: 10.1109/10.301727. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X O, Brown M W, Aggleton J P. Eur J Neurosci. 1995;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 34.Baker R J, Nelder J A. The GLIM (Generalised Linear Interactive Modelling) System. Numerical Algorithm Group 70, Oxford: Royal Statistical Society; 1978. [Google Scholar]
- 35.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 36.Malenka R, C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 37.Wallenstein G V, Eichenbaum H, Hasselmo M E. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 38.Huganir R L, Greengard P. Neuron. 1990;5:555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- 39.Kim J H, Huganir R L. Curr Opin Cell Biol. 1999;11:248–254. doi: 10.1016/s0955-0674(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 40.MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 41.Hennevin E, Hars B, Maho C, Bloch V. Behav Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- 42.Plihal W, Born J. J Cognit Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 43.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 44.Smith C. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 45.Stickgold R. Trends Cognit Sci. 1998;2:484–492. doi: 10.1016/s1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- 46.Plihal W, Born J. NeuroReport. 1999;10:2741–2747. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 47.McGaugh J L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 48.Solodkin M, Cardona A, Corsi-Cabrera M. Physiol Behav. 1985;35:343–348. doi: 10.1016/0031-9384(85)90306-3. [DOI] [PubMed] [Google Scholar]
- 49.Patterson T A, Rose S P R. Behav Neurosci. 1992;106:467–470. doi: 10.1037//0735-7044.106.3.465. [DOI] [PubMed] [Google Scholar]
- 50.Squire L R, Alvarez P. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 51.Shadmehr R, Holcomb H H. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]