Figure 2.
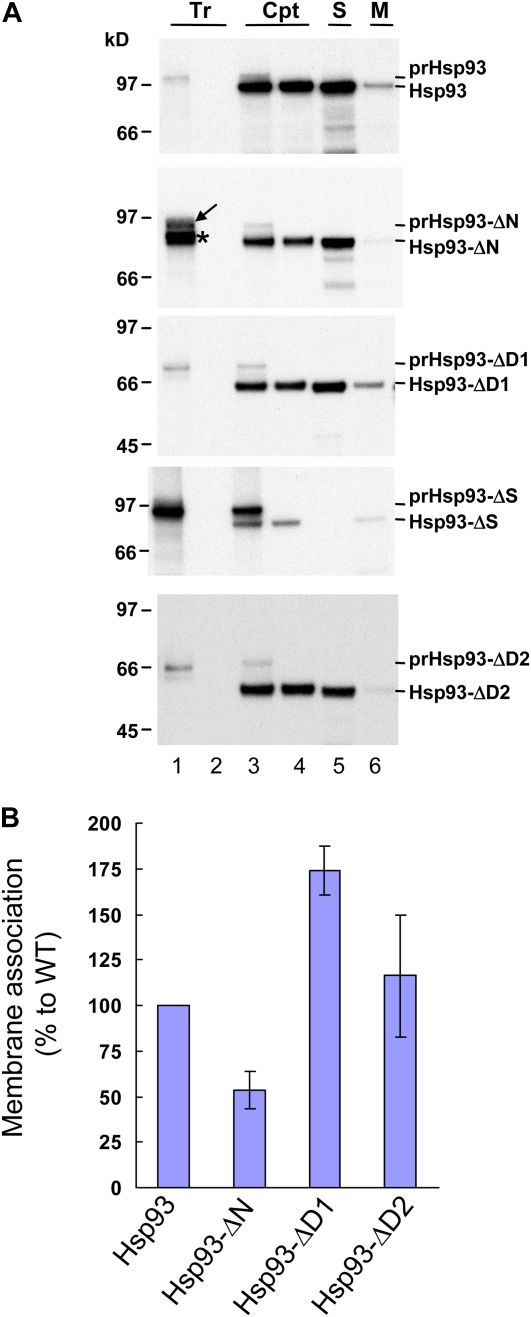
The Hsp93 N domain is important for Hsp93 membrane association. A, In vitro-translated [35S]Met-prHsp93 and various deletion mutants (Tr; lane 1 of all panels) were treated with thermolysin directly (lane 2) or incubated with isolated pea chloroplasts (Cpt) under import conditions for 25 min. After import, a small portion of the chloroplasts were centrifuged through a 40% Percoll cushion to reisolate intact chloroplasts (lane 3). The rest of the reactions were pelleted and resuspended, and the chloroplasts were further digested with thermolysin. After digestion, intact chloroplasts were reisolated through a 40% Percoll cushion (lane 4). Some chloroplasts were further lysed hypotonically and separated into soluble (S; lane 5) and membrane (M; lane 6) fractions by centrifugation. The proteins in the soluble fraction were precipitated by TCA and dissolved with the protein extraction buffer. The membrane fractions were resuspended with the same volume of protein extraction buffer. Protein concentrations were then determined for all samples. Ten micrograms of proteins was loaded in all the chloroplast and soluble fraction lanes (lanes 3–5). For each membrane fraction, the same volume as its corresponding soluble fraction was loaded, so each pair of membrane and soluble fractions shown were derived from the same amount of chloroplasts. Samples were analyzed by SDS-PAGE and fluorography. All Tr lanes contain 20% of the in vitro-translated precursors used for the chloroplast (Cpt) lanes. B, The amount of imported mature proteins in the soluble and membrane fractions (lanes 5 and 6 of A) was quantified. The percentage in the membrane for each construct was calculated and then normalized to the wild type (WT). Data shown are means ± sd (n = 3). Data for prHsp93-ΔS were not included because no signal was obtained in the soluble fraction. [See online article for color version of this figure.]