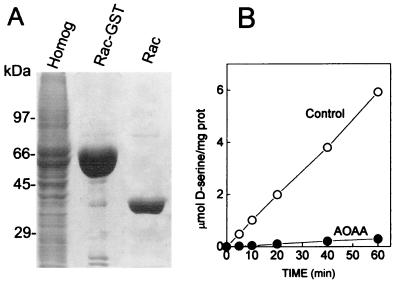
Figure 1.
Purification of active recombinant serine racemase from mammalian cells. Mouse serine racemase-GST fusion protein was expressed in HEK293 cells and purified to homogeneity. (A) SDS/PAGE (12%) stained with Coomassie brilliant blue. Lane 1, 40 μg transfected HEK293 cell protein extract; lane 2, 30 μg mouse serine racemase-GST fusion protein purified with glutathione-Sepharose beads; lane 3, 5 μg purified serine racemase after GST portion was removed by cleavage with thrombin. (B) Serine racemase activity was measured at 37°C in media containing 50 mM Tris⋅HCl (pH 8.2), 10 μg/ml recombinant serine racemase, 15 μM PLP, and 10 mM l-serine. Recombinant serine racemase exhibited robust racemase activity (○), which was totally inhibited by 1 mM AOAA (●). The data are representative of six different purifications.