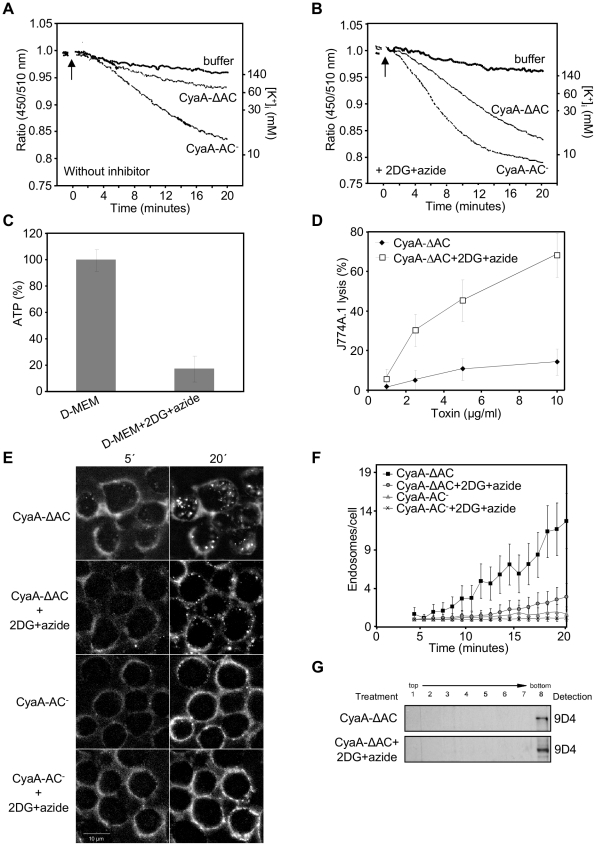
Figure 7. Rapid removal from the plasma membrane reduces toxoid-mediated K+ efflux from monocytes.
(A) PBFI/AM loaded J774A.1 monocytes in HBSS buffer were exposed to 3 µg/ml of enzymatically inactive CyaA-AC− toxoid, or to its CyaA-ΔAC variant, and K+ efflux at 25°C was monitored in time as described under Materials and Methods. (B) PBFI/AM loaded J774A.1 macrophages were preincubated for 15 minutes in HBSS buffer containing 0.01% sodium azide and 10 mM 2DG prior to addition of CyaA-AC− or CyaA-ΔAC proteins and time course of K+ efflux was recorded. The shown curves are representative of six independent experiments. (C) 106 J774A.1 cells were incubated for 15 minutes in D-MEM or in D-MEM containing the glycolysis inhibitor 2-deoxy-D-glucose (2DG, 10 mM) instead of glucose and 0,01% sodium azide. ATP content in cells was determined using the ATP Bioluminescence Assay Kit CLS II (Roche). (D) Prior to addition of the CyaA-ΔAC protein, 2×105 J774A.1 macrophages were preincubated for 15 minutes at 37°C in D-MEM alone or in D-MEM medium without glucose and containing 0.01% sodium azide plus 10 mM 2DG. The extent of cell lysis was determined using the Cytotox 96 assay kit (Promega) as the amount of lactate dehydrogenase released into culture media in 2 hours. Average values from three independent experiments performed in duplicates are shown. (E) CyaA-ΔAC, in contrast to CyaA-AC− is rapidly internalized into J774A.1 cells. J774A.1 cells were preincubated for 15 minutes in HBSS, or in glucose-free HBSS with 2DG and sodium azide, before 5 µg/ml of Fluor 488-labeled CyaA-ΔAC or CyaA-AC− were added. Toxoid endocytosis was analyzed by live cell imaging and (F) Numbers of endosomes containing CyaA-ΔAC and CyaA-AC− were determined as above. (G) J774A.1 cells were preincubated for 15 minutes in HBSS or in HBSS containing sodium azide and 2DG prior to addition of CyaA-ΔAC (500 ng/ml). Detergent-resistant membrane microdomains (DRMs) were extracted, separated and probed as in Figure 1B.