Table 3.
SAR around hit series 1 b.
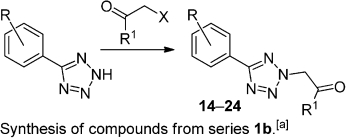 | |||
|---|---|---|---|
| Compd | R | R1 | TbTryS IC50 [μm] |
| 14 | H | tBu | 8.8 |
| 15 | 4-CF3 | tBu | >100 |
| 16 | 3-Cl, 4-CH3 | tBu | 7.2 |
| 17 | 3-F | tBu | 1.5 |
| 18 | 3-CF3 | tBu | 3.5 |
| 19 | 3,5-di-F | tBu | 1.9 |
| 20 | 3,5-di-Cl | tBu | 0.3 |
| 21 | 3,5-di-Cl | CH3 | 6.5 |
| 22 | 3,5-di-Cl | CH2CH3 | 2.7 |
| 23 | 3,5-di-Cl | N(CH3)2 | 0.6 |
| 24 | 3,5-di-F | Ph | 45 |
[a] Conditions: NaI, K2CO3, DMF, 90 °C; yields: 44–86 %; the synthesis of compound 20 was previously described by Torrie et al.20
