Figure 1.
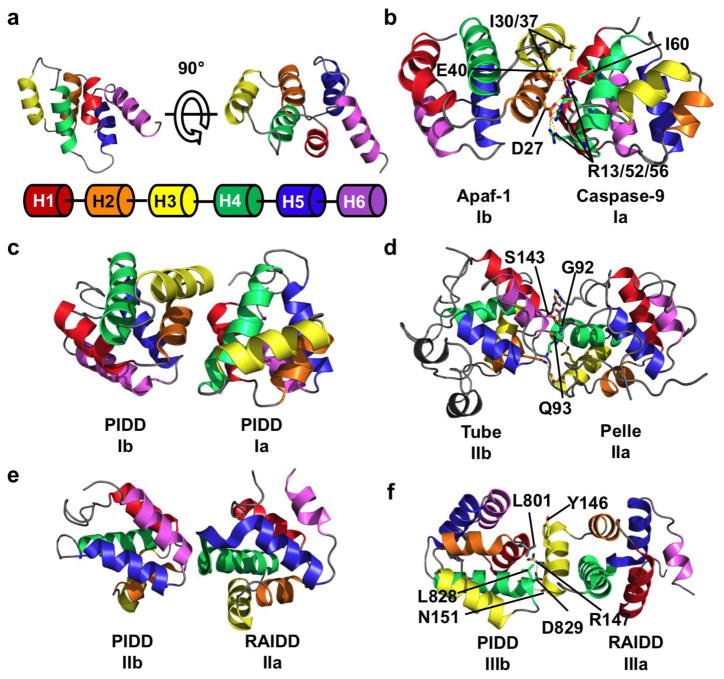
Three types of asymmetric interactions mediate homotypic DD binding. (a) NMR structure of the DD of Fas, showing the six helical bundle domain architecture of the DD superfamily. Coloring scheme is shown below the structure and used throughout the figure. (b) Crystal structure of the heterodimer between the CARD domain of Apaf-1 and Caspase-9. In this type I interaction, negatively charged residues from H2 and H3 (interface Ib) of Apaf-1 interact with positively charged H1 and H4 (interface Ia) of Caspase-9. A hydrogen bond network formed by D27/E40 of Apaf-1 and R13/R52/R56 of Caspase-9 contributes to the interaction. Hydrophobic interactions between Apaf-1 I30/I37 and Caspase-9 I60 also contribute. (c) Type I interaction between two PIDD DDs. The interface architecture is highly similar to that of Apaf-1/Caspase-9. (d) Crystal structure of Tube and Pelle DD heterodimer showing a type II interface. The main chain amide of Tube S143 forms a pseudo α-helical hydrogen bond with the carbonyl of Pelle Q93. The side chain hydroxyl of S143 forms an addition hydrogen bond with the main chain carbonyl of Pelle G92. Several charge-charge interactions also contribute to binding. The interaction between the C-terminal tail of Tube and Pelle is not shown. (e) Type II interaction between PIDD and RAIDD DDs. The C-terminal end of RAIDD H4 and the H4–H5 loop (interface IIa) interacts with the N-terminal end of PIDD H6. (f) Type III interaction between PIDD and RAIDD DDs. H3 of RAIDD (interface IIIa) interacts with the H1–H2 and H3–H4 loops (interface IIIb) of PIDD. A salt-bridge between PIDD D829 and RAIDD R147, a hydrogen bond between PIDD L828 and RAIDD N151, and a hydrophobic interaction between PIDD L801 and RAIDD Y146.