Figure 4.
A Conserved Patch in the DS Domain Is Required for DDR1 Signaling
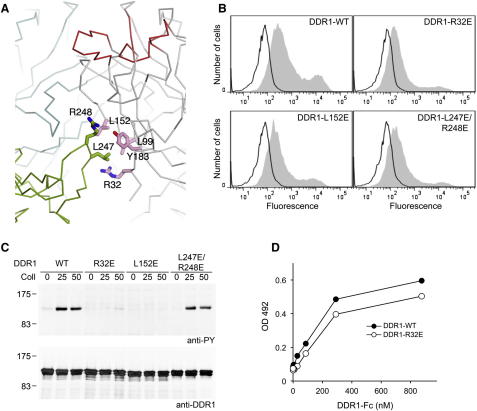
(A) The lattice contact resulting in a symmetric DDR1 dimer (see text). The DDR1 molecule on the left is in cyan (DS domain) and green (DS-like domain); the DDR1 molecule on the right is in gray, with the collagen-binding loops (Carafoli et al., 2009) in red. The 2-fold symmetry axis is vertical. Selected residues are shown in atomic detail (pink, conserved surface patch in the DS domain).
(B) Cell surface expression of mutants. Wild-type DDR1b or the indicated mutants were transiently expressed in HEK293 cells. The cells were stained on ice with 10 μg/ml of anti-DDR1 mAb 7A9 (filled gray histograms) or mouse IgG1 isotype control Ab (black lines) followed by FITC-conjugated goat-anti mouse IgG and analysis by flow cytometry. The experiment was performed twice with similar results.
(C) Collagen-induced activation of mutants. Wild-type DDR1b or the indicated mutants were transiently expressed in HEK293 cells. The cells were stimulated with collagen I at the indicated concentrations (in μg/ml). Aliquots of cell lysates were analyzed by SDS-PAGE and western blotting. The blots were probed with anti-phosphotyrosine (anti-PY) mAb 4G10 (upper blot) and reprobed with anti-DDR1 Abs (lower blot). The experiment was performed three times with similar results.
(D) Solid-phase binding assay with recombinant DDR1-Fc protein (filled circles, wild-type; open circles, R32E mutant) added to 96-well plates coated with collagen peptide III-23 (Xu et al., 2011). Bound DDR1-Fc was detected with anti-human Fc Ab and was measured as absorbance at 492 nm. Shown is a representative of two independent experiments, each performed in duplicate.