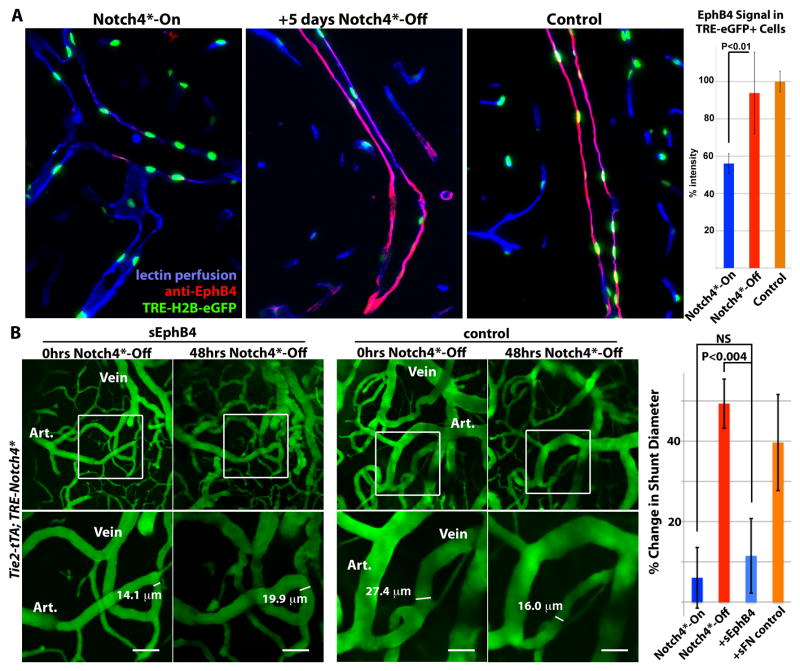
Figure 5. Venous marker EphB4 is re-expressed in venous endothelial cells and required for AV shunt regression.
(A) Sagittal sections showing veins in the cerebellum of Tie2-tTA; TRE-Notch4*; TRE-H2B-eGFP mutants before and 5 days after Notch4* repression, with littermate Tie2-tTA; TRE-H2B-eGFP control. EphB4 expression in TRE-eGFP+ cells, by immuno-fluorescence staining, was selectively reduced in Notch4* expressing mutant, and recovered upon Notch4* repression. Graph shows quantification of EphB4 fluorescence signal intensity in TRE-GFP+ cells. n=4 for mutants, n=3 for controls, an average of ~12 cells per vessel and >5vessels per mouse. (B) Two-photon timelapse imaging of cortical brain vessels through a cranial window in Notch4* mutant mice. Plasma was labeled by intravenous FITC-dextran. Treating Notch4* mutant mice with soluble EphB4 (sEphB4) inhibited the regression of the AV shunt examined over 48hrs of Notch4* repression. In a littermate Notch4* mutant treated with control soluble human fibronectin (sFN), the AV shunt was reduced in diameter following 48hrs of Notch4* repression. Quantification of changes in minimal AV shunt diameter over 48hrs in mice without repression of Notch4* (Notch4*-On, n=35 AV shunts in 11 mice), with repression of Notch4* (Notch4*-Off, n=22 AV shunts in 10 mice), with repression of Notch4* and sEphB4 intravenous treatment (+sEphB4, n=26 AV shunts in 5 mice), and with repression of Notch4* and sFN control intravenous treatment (+sFN control, n=13 AV shunts in 2 mice). Error bars represent s.e.m. between individual AV shunts.