Abstract
Background
Studies of pediatric intensive care unit (PICU) patients have shown a significant association of morbidity and mortality with hyperglycemia. We retrospectively evaluated the degree of hyperglycemia as well as its correlation with glucocorticoid and insulin use and assessed its association with hospital length of stay (LOS) and mortality. This study preceded the initiation of a standard glycemic control protocol.
Methods
We examined medical records at Kosair Children's Hospital for all PICU admissions from 2008 of patients without diabetes mellitus. Critical illness hyperglycemia (CIH) was defined by having three or more peak glucose values greater than thresholds of 110, 140, 180, and 200 mg/dl. These patients were evaluated for glucocorticoid, insulin use, and outcome measures.
Results
We evaluated the eligible 1173 admissions, where 10.5% of these patients reached the highest threshold (200 mg/dl) of CIH. Glucocorticoids were used in 43% of these patients, with dexamethasone being the most common (58%). There was a significant correlation between glucocorticoids and higher peak glucose values, where 81% of the patients who were above the 200 mg/dl cutoff level were treated with glucocorticoids. Only 36.8% in that group were also treated with insulin. Patients at the 200 mg/dl cutoff had the highest median PICU and total hospital length of stays (4 and 10 days, respectfully). Mortality was associated with increasing glucose levels, reaching 18.7% among patients above the 200 mg/dl cutoff.
Conclusion
Hyperglycemia was prevalent in the PICU and was associated with increased morbidity, as characterized by increased LOS and increased mortality. Glucocorticoid use was prevalent among patients exhibiting hyperglycemia. Insulin use was uncommon.
Keywords: critical illness, glucocorticoids, glucose, hyperglycemia, hypoglycemia, insulin, intensive care, morbidity, mortality, pediatric
Introduction
Glycemic variability, most notably hyperglycemia, has been associated with increased morbidity and mortality in both adult and pediatric intensive care unit (PICU) patients with and without diabetes mellitus.1–5 More specifically, hyperglycemia is associated with adverse outcomes in a variety of clinical settings in children and adults including trauma,6,7 head injury,8–14 burn injury,15 cardiac,16–20 and post-operative patients.21–23 Large, prospective trials using insulin therapy to control hyperglycemia in adult medical and surgical intensive care units (ICU), however, have displayed conflicting results regarding reduction in morbidity and mortality.22,24–28
These apparent contradictions may be explained in part by differences in study protocols, patient populations, glycemic targets, rates of hypoglycemia, nutrition, glucose monitoring, and the use of agents influencing glycemic variability and control (e.g., including insulin and glucocorticoids).29,30 A meta-analysis of 26 trials, including the Leuven studies and the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial, concluded that mortality is reduced in some patients, namely surgical, but not in all ICU patients treated using intensive glycemic goals (frequently defined at targets of 80–110 mg/dl).31
In addition to the influences of physiologic stresses on carbohydrate metabolism, medications such as glucocorticoids affect production and utilization of glucose. Glucocorticoid use for ICU patients is prevalent and administered for a variety of clinical indications. In the 2006 study reported by Van den Berghe and collegues24, nearly 54% of medical ICU subjects received glucocorticoids. These were used primarily for immuno-suppression or as anti-inflammatory agents. In the NICE-SUGAR trial, septic shock was the most common reason for treatment with glucocorticoids in more than 33% of subjects.27 Although there appeared to be a treatment-specific effect with the use of glucocorticoids at baseline in the NICE-SUGAR trial (p = 0.6), neither study specifically showed a direct influence on mortality.
Less is known about the incidence of glucocorticoid use and its correlation with hyperglycemia in the pediatric intensive care unit (PICU). In a retrospective study of postoperative cardiac ICU patients, glucocorticoid use was higher among children with hyperglycemia versus those without (36% versus 18%, p < .05).32 However, there has not been a study that more broadly examines the associations of glucocorticoids and their potential impact on glycemic variability in children in a PICU. We performed a retrospective analysis of all pediatric patients admitted to our PICU over a 12-month period to evaluate the associations of varying thresholds of hyperglycemia and of insulin and glucocorticoid use with morbidity (hospital length of stay) and mortality in a PICU population.
Methods
This retrospective analysis was conducted using data extracted from computerized medical records data at Kosair Children's Hospital in Louisville, Kentucky (MEDITECH, Medical Information Technology, Inc.). A limited data set from all PICU admissions (medical and surgical) from January 1, 2008 to December 31, 2008 was obtained. For patients with multiple PICU admissions, only the last admission was used to assess associations with mortality more accurately. Patients ≥19 years of age, those with a diagnosis of diabetes mellitus, or those who had no glucose values during their PICU stay were excluded. Receipt of glucocorticoids and insulin, primary diagnosis ICD-9 code (International Statistical Classification of Diseases and Related Health Problems, Ninth Revision), and general demographic information were available. During the study period, there was no glucose management protocol in place; therefore, all glucose measurements were obtained and treated at the discretion of managing physicians. Pediatric ICU length of stay (LOS), overall hospital LOS, and mortality were also available. This study was approved by the Institutional Review Board of the University of Louisville.
Glucose Parameters
Three methods for measuring glucose were used: plasma glucose via the hospital clinical laboratory (Olympus AU640/AU2700, Olympus America, Inc., Center Valley, PA), whole blood glucose obtained in association with blood gas monitoring (Radiometer ABL800 FLEX analyzer, Radiometer Medical, Bronshoj, Denmark), and whole blood glucose via two point of care devices (Accu-Chek Inform, Roche Diagnostics, Indianapolis, IN; Abaxis i-STAT 200 Analyzer, Abaxis, North America, Union City, CA). Quality assurance for all three of these methods followed standard hospital laboratory procedures.
Cutoff values for both hypoglycemia (65 mg/dl) as well as several hyperglycemia thresholds (110, 140, 180, and 200 mg/dl) were established based on clinical observational and interventional pediatric and adult studies.1,5,18,22,33–35
All oral, intramuscular, and intravenous glucocorticoids were included in the analyses. Inhaled glucocorticoids were excluded as they have significantly lower systemic bioavailability. All insulin use was included in the analyses. Patient data was used to assess the association between glucose thresholds, glucocorticoid use, PICU LOS, total hospital LOS, and mortality. Insulin use was evaluated both with and without glucocorticoids.
Statistical Analysis
Data analysis was performed using IBM® SPSS® (Version 19.0. IBM Corporation, Armonk, NY). Univariate associations were determined using Chi square, Spearman correlation, and Mann-Whitney U tests. The relation between death, maximum glucose threshold, and minimum glucose threshold were modeled by multiple logistic regression analysis. A stepwise backward conditional approach was used. Variables were entered based on a priori considerations and statistical significance in univariate analyses.
Results
Patients
There were a total of 1653 patient admissions to the PICU at Kosair Children's Hospital from January 1, 2008 to December 31, 2008. Of these patients, 82 (5%) carried a known or new diagnosis of diabetes mellitus, 372 (23%) had no glucose value obtained during their PICU stay, and 26 were 19 years of age or older. After excluding these patients, 1173 patient admissions met inclusion criteria for analysis (Table 1).
Table 1.
Characteristics of Total and Included Patients versus Patients Excluded from Analyses
| Characteristic | All | Included | Excluded | p value |
|---|---|---|---|---|
| n | 1653 | 1173 | 480 | |
| Age (mean ± SD) [median] | 6.6 ± 6.2 [4.3] | 6.0 ± 6.0 [3.4] | 8.0 ± 6.4 [6.8] | <0.001 |
| Gender (%) | 0.252 | |||
| Female | 737 (45) | 534 (45) | 203 (42) | |
| Male | 916 (55) | 639 (55) | 277 (58) | |
| Race/Ethnicity (%) | <0.001 | |||
| White | 1167 (71) | 852 (72) | 315 (66) | |
| Black | 335 (20) | 208 (18) | 127 (26) | |
| Hispanic | 58 (3) | 43 (4) | 15 (3) | |
| Other | 93 (6) | 70 (6) | 23 (5) | |
| PICU LOS (mean ± SD) [median] | 2.6 ± 8.2 [1] | 3.3 ± 9.6 [1] | 0.7 ± 1.5 [0] | <0.001b |
| Hospital LOS (mean ± SD) [median] | 7.3 ± 11.5 [4] | 8.8 ± 13.1 [5] | 3.6 ± 3.9 [4] | <0.001b |
| Deaths (rate %) | 38 (2.3%) | 36 (3.1%) | 2 (0.4%)a | .002c |
SD, standard deviation; LOS, length of stay in days.
Both deaths among excluded patients were ≥19 years old; neither had diabetes.
Chi square test (continuity correction used for 2 × 2 tables).
Mann-Whitney U test.
Patient Demographics, Length of Stay, and Mortality
Of the 1173 eligible patient admissions, 55% (n = 639) were males and children less than 5 years of age making up the largest percentage of eligible admissions (60.1%) by age group (range 0–19 years, median 3.4 years). The median PICU LOS was 1 day and the median total hospital LOS was 5 days. A total of 36 patients of the eligible 1173 (3.1%) died during the study period (Table 1). Of those who died, congenital heart disease (19.4%) was the most common diagnosis involving 2 or more patients, followed by sepsis (16.7%), lung disease not otherwise specified (NOS) (13.9%), cardiac dysrhythmia (5.6%), leukemia (5.6%), and epilepsy (5.6%). The most common diagnoses for all eligible admissions are listed in Table 2.
Table 2.
Fifteen Most Frequent Primary Diagnoses in Included and Excluded Patients
| 3-digit ICD-9 code | Included | 3-digit ICD-9 code | Excluded | ||
|---|---|---|---|---|---|
| Description | Frequency n (%) | Description | Frequency n (%) | ||
| 493 | Asthma | 70 (6.0) | 493 | Asthma | 169 (35) |
| 745 | Congenital bulbus Cordis/Cardiac septal anomaly | 63 (5.4) | 250 | Diabetes mellitus | 82 (17) |
| 486 | Pneumonia | 48 (4.1) | 756 | Congenital musculoskeletal anomaly NOS | 15 (3.1) |
| 345 | Epilepsy | 44 (3.8) | 486 | Pneumonia | 13 (2.7) |
| 518 | Lung disease NOS | 41 (3.5) | 466 | Bronchitis/Bronchiolitis | 12 (2.5) |
| 466 | Bronchitis/Bronchiolitis | 38 (3.2) | 800 | Skull fracture (vault) | 12 (2.5) |
| 737 | Spine curvature | 38 (3.2) | 464 | Acute laryngitis/Tracheitis | 11 (2.3) |
| 746 | Congenital heart disease NOS | 37 (3.2) | 852 | Subarachnoid, subdural, extradural hemorrhage | 11 (2.3) |
| 747 | Congenital circulatory anomaly | 29 (2.5) | 801 | Skull fracture (vault) | 10 (2.1) |
| 996 | Procedure complication | 27 (2.3) | 741 | Spina bifida | 7 (1.5) |
| 038 | Septicemia | 24 (2.0) | 996 | Procedure complication | 7 (1.5) |
| 801 | Skull fracture (base) | 23 (2.0) | 345 | Epilepsy | 6 (1.3) |
| 800 | Skull fracture (vault) | 20 (1.7) | 474 | Chronic tonsil/Adenoid disease | 5 (1.0) |
| 754 | Congenital musculoskeletal deformity | 17 (1.4) | 348 | Brain condition NOS | 4 (0.8) |
| 779 | Perinatal condition NOS | 17 (1.4) | 851 | Cerebral laceration/Contusion | 4 (0.8) |
| Cumulative of all eligible cases | 536 (45.7) | Cumulative of all excluded cases | 368 (76.7) | ||
General Glucose Data
Hyperglycemia (three or more values >110 mg/dl) and hypoglycemia (one or more values <65 mg/dl) were common in our subject population (Table 3). Nearly a quarter of all patients had three or more glucose values >140 mg/dl and 16.9% of patients had at least one value <65 mg/dl. Among the latter, 30.7% also had three or more values >200 mg/dl (Table 3).
Table 3.
Hospital Length of Stay and Mortality for Patients With and Without Three or More Glucose Values Above Each Glucose Cutoffa
| Glucose cutoff (mg/dl) | n (% of total population) | Total hospital LOS, days (mean ± SD) [median] | PICU LOS, days (mean ± SD) [median] | Mortality rate (%) |
|---|---|---|---|---|
| <110 | 701 (59.8) | 5.6 ± 6.0 [3.2] | 1.3 ± 2.7 [1.0] | 2 (0.3) |
| >110 | 472 (40.2) | 13.6 ± 18.3 [7.5] | 6.4 ± 14.2 [2.0] | 34 (7.2) |
| <140 | 894 (76.2) | 6.6 ± 7.1 [4.0] | 1.7 ± 3.6 [1.0] | 7 (0.8) |
| >140 | 279 (23.8) | 16.0 ± 22.2 [8.0] | 8.5 ± 17.6 [3.0] | 29 (10.4) |
| <180 | 1009 (86.0) | 7.2 ± 8.7 [5.0] | 2.3 ± 6.0 [1.0] | 10 (1.0) |
| >180 | 164 (14.0) | 19.1 ± 25.3 [9.0] | 10.0 ± 19.6 [4.0] | 26 (15.9) |
| <200 | 1050 (89.5) | 7.4 ± 9.3 [5.0] | 2.4 ± 5.9 [1.0] | 13 (1.2) |
| >200 | 123 (10.5) | 21.0 ± 27.1 [10.0] | 11.8 ± 22.2 [4.0] | 23 (18.7) |
| All | 1173 (100) | 8.8 ± 13.1 [5] | 3.3 ± 9.6 [1] | 36 (3.1) |
LOS, length of stay.
All p values < 0.001 for each comparison (group with higher versus lower values for each glucose threshold) for each outcome. Mann Whitney test for continuous outcomes, Chi square for categorical.
Pediatric ICU and hospital LOS and mortality rates by various glucose thresholds are shown in Table 3. Those with any hypoglycemia (<65 mg/dl) had a mortality rate of 13.2% versus 1.1% for those who did not have a low glucose value (p < .001). Additionally, patients with at least one glucose value <65 mg/dl plus three or more values >200 mg/dl had a mortality rate of 30% (19 deaths). Crude descriptive mortality rates for one, two, and three glucose values above the 110, 140, 180, and 200 mg/dl thresholds are shown in Table 4.
Table 4.
Mortality by Number of Glucose Values above Cutoff
| # above cutoff | >110 mg/dl % (n) | >140 mg/dl % (n) | >180 mg/dl % (n) | >200 mg/dl % (n) |
|---|---|---|---|---|
| 1 | 4.1 (35) | 5.7 (34) | 8.3 (30) | 9.8 (29) |
| 2 | 5.8 (35) | 8.5 (32) | 12.7 (29) | 14.5 (26) |
| 3 | 7.2 (34) | 10.4 (29) | 15.9 (26) | 18.7 (23) |
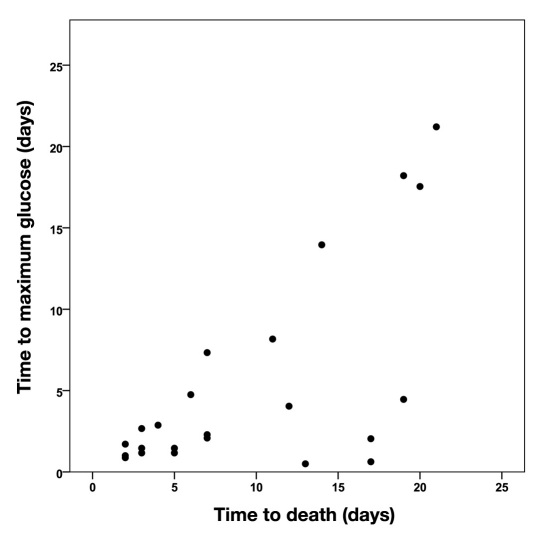
Among the 36 patients who died, time to maximum glucose value and time to death (LOS) was highly correlated (Spearman correlation coefficient = 0.77, p < .001). Among 23 patients who died 2–25 days after hospitalization, the Spearman coefficient was 0.56 (p = .006; Figure 1).
Figure 1.
Correlation of values of time to maximum glucose and time to death for those dying after 1 day and before 25 days. Spearman correlation coefficient = .56, p = .006.
Glucose and Insulin Use
Despite significant hyperglycemia, insulin use was uncommon with only 50 (4.3%) subjects receiving it (Table 5). This was prior to the adoption of a standard approach to hyperglycemia detection and management. Of the 50 patients, 38 (76%) received insulin infusions and 12 (24%) received a subcutaneous or intravenous bolus. Multivariate logistic regression showed no difference with mean age, gender, or race for insulin use. All 50 insulin treated patients had three or more glucose values >140 mg/dl, but those patients using insulin accounted for only 21.8% of those with values at or above this threshold. Insulin use frequency did increase with increasing maximum glucose threshold, but was still only used in 29.3% with three or more values >180 mg/dl and 36.6% of patients with values >200 mg/dl (p < .001). Of patients having one or more glucose values <65 mg/dl (n = 189), only 17.5% (n = 33) were treated with insulin (p = .024). However, this also means that 66% of patients who received insulin had one or more episodes of hypoglycemia.
Table 5.
Mortality, Length of Stay, and Glucose Threshold with and without Insulina
| n | LOS, days | Glucose thresholdsb | ||||||
|---|---|---|---|---|---|---|---|---|
| PICU (mean ± SD) [median] | Total hospitalc (mean ± SD) [median] | <65 mg/dldn (%) | >110 mg/dl n (%) | >140 mg/dl n (%) | >180 mg/dl n (%) | >200 mg/dl n (%) | ||
| No insulin | 1123 | 2.6 ± 6.4 [1] | 8.0 ± 10.9 [5] | 156 | 422 | 229 | 116 | 78 |
| Survived | 1102 | 2.6 ± 6.4 [1] | 8.0 ± 10.9 [5] | 145 (92.9) | 403 (95.5) | 215 (93.9) | 105 (90.5) | 70 (89.7) |
| Died | 21 | 4.5 ± 3.8 [3] | 6.1 ± 6.1 [4] | 11 (7.1) | 19 (4.5) | 14 (6.1) | 11 (9.5) | 8 (10.3) |
| Insulin | 50 | 19.1 ± 31.3 [9] | 26.5 ± 32.4 [16] | 33 | 50 | 50 | 48 | 45 |
| Survived | 35 | 11.9 ± 14.8 [8] | 21.2 ± 19.0 [13] | 19 (57.6) | 35 (70) | 35(70) | 33 (68.8) | 30 (66.6) |
| Died | 15 | 36.1 ± 49.6 [17] | 38.9 ±50.7 [17] | 14(42.4) | 15 (30) | 15 (30) | 15 (31.2) | 15 (33.3) |
| Total | 1173 | 3.3 ± 9.6 [1] | 8.8 ± 13.1 [5] | 189 (16.1) | 472 (40.2) | 279 (23.8) | 164 (14.0) | 123 (10.5) |
LOS, length of stay.
All p values < 0.001 for insulin versus no insulin variables, except where indicated.
In patients with three or more blood glucose measurements obtained at each cutoff.
p value > 0.05.
Patients with one or more values <65 mg/dl.
Both LOS and mortality were associated with insulin use. There was a tendency toward longer PICU and total hospital LOS in patients who received insulin versus those who did not. When evaluating mortality with or without insulin use for each glucose cutoff, there was a significant association between insulin use and death at each cutoff (Table 5). Patients with hypoglycemia had an even higher mortality rate with insulin use. Overall, 15 of the 50 patients (30%) who used insulin died. However, when looking at total deaths, the majority (58.3%) were in patients who did not receive insulin.
Glucose and Glucocorticoids
One or more glucocorticoid agents were administered to 43.1% (n = 505) of eligible patients (Table 6). Dexamethasone was used in 25%, followed by methylprednisolone (16.3%), prednisone (15.5%), and hydrocortisone (3.3%). The specific indication for each glucocorticoid was not available for this analysis. In general, however, dexamethasone is primarily used prior to extubation, and methylprednisolone is used for asthma and in patients with significant lung disease. Prednisone is usually used orally in children with lower acuity, and hydrocortisone is primarily used for septic or acutely ill, unstable patients. Although patients treated with hydrocortisone tended to be younger (mean 4.4 years versus 6.1 years; p = .003) versus those not treated, age was not a factor for glucocorticoid use overall.
Table 6.
Percentage of Patients with and without Glucocorticoid Use by Glucose Cutoffa
| n | <65 mg/dlbn (%) | >110 mg/dl n (%) | >140 mg/dl n (%) | >180 mg/dl n (%) | >200 mg/dl n (%) | |
|---|---|---|---|---|---|---|
| Dexamethasone | 293 | 82 (28.0) | 203 (69.3) | 146 (49.8) | 92 (31.4) | 69 (23.5) |
| No dexamethasone | 880 | 107 (12.2) | 269 (30.6) | 133 (15.1) | 72 (8.2) | 54 (6.1) |
| Methylprednisolone | 191 | 37 (19.4)c | 104 (54.5) | 71 (37.2) | 48 (25.1) | 38 (19.9) |
| No methylprednisolone | 982 | 152 (15.5)c | 368 (37.5) | 208 (21.2) | 116 (11.8) | 85 (8.7) |
| Prednisone | 182 | 20 (11.0)c | 84 (46.2)c | 51 (28.0)c | 30 (16.5)c | 21 (11.5)c |
| No prednisone | 991 | 169 (17.1)c | 388 (39.2)c | 228 (23.0)c | 134 (13.5)c | 102 (10.3)c |
| Hydrocortisone | 39 | 29 (74.4) | 35 (89.7) | 26 (66.7) | 23 (59.0) | 18 (46.2) |
| No hydrocortisone | 1134 | 160 (14.1) | 437 (38.5) | 253 (22.3) | 141 (12.4) | 105 (9.3) |
| Any glucocorticoid | 505 | 117 (23.2) | 309 (61.2) | 210 (41.6) | 133 (26.3) | 99 (19.6) |
| None | 668 | 72 (10.8) | 163 (24.4) | 69 (10.3) | 31 (4.6) | 24 (3.6) |
In patients with three or more blood glucose measurements obtained at each cutoff. All p values < 0.001, except where indicated.
Patients with one or more values <65 mg/dl.
p value > 0.05.
Glucocorticoid use and critical illness hyperglycemia (CIH) were associated at each glucose cutoff (Table 6). Among patients who received one or more glucocorticoid agents, more than 60% had 3 or more glucose values >110 mg/dl and nearly 20% had values >200 mg/dl. However, the available data did not allow evaluation of timing of glucocorticoid administration with onset of hyperglycemia.
Pediatric ICU and hospital LOS were significantly longer in those treated with glucocorticoids. Compared with all eligible patients, the median total hospital and PICU LOS for patients treated with dexamethasone was 2 and 6 days, respectively. For those who received hydrocortisone, the median PICU LOS was 5 days (p < .001) and total LOS 9 days (p = .002).
Overall, the vast majority (28 of 36; 78%) of patients who died received glucocorticoids (p < .001), but only 5.5% of all children who received a glucocorticoid died. Illustrating this further, 39% of children who died received methylprednisolone, but these children accounted for only 7% of those who received this agent. In contrast, 56% of those who died received hydrocortisone, but this was equivalent to 51.3% of those treated with this agent (p < .001). The mean number of treatment doses for methylprednisolone and hydrocortisone in the deceased were 15.9 and 7.7 compared with 1.8 and 0.3 in survivors, respectively. Of 505 patients who used any glucocorticoid during admission, 41 (8.1%) were also treated with insulin. Of these 41 patients, 48% were given dexamethasone, but these represented only 14% of all children who received dexamethasone.
Risk of Mortality
In a multivariate logistic regression analysis, race, age, gender, PICU LOS, glucose cutoffs, glucocorticoid use (dexamethasone, hydrocortisone, methylprednisolone, and prednisone), and insulin use (any versus none) were included to adjust for their impacts on mortality (p < 0.001–0.030). Pediatric ICU LOS, as opposed to total hospital LOS, was used as this was deemed a more accurate marker of illness severity. Based on our initial univariate analysis of each individual glucose cutoff, values >110 mg/dl, >180 mg/dl, and <65 mg/dl were included.
Stepwise backward modeling revealed that three or more glucose values >110 mg/dl [odds ratio (OR) 7.1, 95% confidence interval (CI) 1.4–36.3; p < 0.001), three or more glucose values >180 mg/dl (OR 45.6, 95% CI 9.3–224.4; p < .001), one or more glucose values <65 mg/dl (OR 3.0, 95% CI 1.1–7.9; p = .02), and the receipt of hydrocortisone (OR 21.0, 95% CI 7.3–60.3; p < .001) were independently associated with mortality. Dexamethasone (OR 0.14, 95% CI 0.05–0.4) and prednisone (OR 0.1, 95% CI 0.02–0.5) were associated with lower risk of death, and thus appeared to be markers for survival.
Race, age, gender, PICU LOS, and insulin use were not independently associated with mortality.
Discussion
Hyperglycemia and glycemic control in the PICU setting has been receiving considerable attention. Several studies have shown an association between glucose and poor outcome in children admitted for both medical and surgical treatment in the PICU.5,15,36–40 Numerous mechanisms for this association have been proposed, including increased inflammatory cytokine production, acute endothelial dysfunction, hypercoagulation, metabolic disturbances, and increased cellular apoptosis.41 While the exact benefit of using insulin therapy remains in question, use of nutritional interventions along with insulin to maintain normoglycemia does appear to be protective.42 Therefore, a number of national oversight committees, including the Institute for Healthcare Improvement, American Diabetes Association, and Society of Critical Care Medicine have recommended routine assessment and treatment of hyperglycemia in critically ill patients.43–45 It is unclear as to what extent these recommendations have been adopted by pediatric intensivists.46,47
By stratifying glucose data according to the number of glucose values above each cutoff, the prevalence of hypo- and hyperglycemia was identified more accurately and the influence of spurious “one-time” glucose measurements was removed. Similar to previous reports, increasing glucose values were associated with increasing total hospital and PICU LOS with the greatest LOS reported in patients above the 200 mg/dl cutoff. Mortality was also significantly elevated with the highest individual mortality rate (18.9%) in patients above the 200 mg/dl cutoff.
After removing patients with a diagnosis of diabetes mellitus, only 50 (4.3%) patients in our institution were treated with insulin during their hospitalization. This inherently limited the ability to analyze the impact of insulin use on LOS or mortality. However, considering the prevalence of hyperglycemia, it appears to provide an example of the wary approach to glucose control in our hospital during this period. While the mortality rate at the varying cutoffs was higher among patients who used insulin versus those who did not, the subject number and database limitation prevent any statements on causality. Of perhaps more interest was the data on hypoglycemia and insulin use. Studies in the adult ICU setting using insulin to control hyperglycemia of critical illness have shown high rates of hypoglycemia with varied correlation with mortality.24,27,48,49 In the PICU, several studies have successfully demonstrated the use of insulin-glucose control protocols without significantly increasing the rate of hypoglycemia.50,51 In a 2008 study by Preissig and colleagues51, the overall rate of severe hypoglycemia (<40 mg/dl) was 4% in those receiving insulin for CIH.51 The rate of less severe hypoglycemia (<60 mg/dl) was actually found to be higher in patients who did not receive insulin (7%) versus those who did (6.7%). This risk, though, is clearly protocol-dependent as demonstrated by a 25% hypoglycemia rate (<40 mg/dl) reported by Vlasselaers and colleagues52 in children treated with intensive management versus conventional therapy (1%). Although prolonged, severe hypoglycemia strongly correlates with increased risk of morbidity and mortality, a prospective pediatric study has yet to evaluate the impact of short-term hypoglycemia on outcome.
Glucocorticoids are widely used in the treatment of critical illness for a variety of proven and unproven indications. Their relationship with glucose homeostasis has been shown to be caused by a combination of mechanisms, including direct inhibition of insulin secretion, a reduction of insulin sensitivity, and impairment of glucagon production.53–55 While the majority of studies report on treatment of specific conditions, such as septic shock,56 many large-scale prospective trials provide limited details regarding their use. In an observational study by Weiss and colleagues,57 of 804 surgical patients admitted to the ICU, 33% were treated with glucocorticoids. Surprisingly, glucocorticoids were positively associated with an increased risk of hypoglycemia (<80 mg/dl) as well as hyperglycemia (>150 mg/dl).57 In the NICE-SUGAR trial, 33% of patients were treated with unspecified glucocorticoids with septic shock being the most common reason identified.27 This contrasts sharply to Van den Berghe's study in which a much greater percentage (54%) of patients were treated with glucocorticoids; the majority used methylprednisolone (75%), followed by hydrocortisone (38%), and dexamethasone (.01%).24 Neither of these studies, however, provided specific details regarding glucocorticoid treatment and the impact on glycemia. It is unclear if glucocorticoids (specific types or general) are used in ICU patients for specific indications or are a marker of late-state therapeutic desperation.
In pediatrics, the prevalence of glucocorticoid use and its association with blood glucose in the PICU is even less well defined. Studies in specific pediatric populations, such as cardiac surgical patients,58 demonstrated a significant correlation between glucocorticoids and CIH. Our analysis showed a high prevalence of glucocorticoid use (43.1%) and a strong statistical association with hypoglycemia and hyperglycemia, as well as increased LOS and mortality. The correlation with increased LOS and risk of mortality was most pronounced with hydrocortisone usage. This is, perhaps, not surprising in that the use of hydrocortisone is often relegated to the most severely ill patients, regardless of age. Dexamethasone was used in only 5 of 644 patients in the medical ICU reported by Van den Berghe, but it was the most commonly used steroid in our study (25%).24 Interestingly, its use correlated with reduced mortality.
Although our limited data set did not provide treatment specifics, we speculate that its use at the time of extubation may at least partially explain this reduction. Despite the decreased mortality, dexamethasone, like hydrocortisone, was highly associated with both hypo- and hyperglycemia. While we do not have the data to provide a causal explanation for the mortality disparity between glucocorticoids, several studies in critically ill adults suggest an increased risk of mortality with high doses of glucocorticoids.59,60 These coincide with the observational findings of several authors linking high serum cortisol levels with poor outcome.61–63 Furthermore, lowering cortisol levels appears to be associated with improved survival. In 451 adult ICU patients randomized to intensive versus conventional insulin treatment, Vanhorebeek and collegues64 showed a significant survival benefit with lowering free cortisol levels from baseline. This benefit was greatest in patients receiving intensive insulin therapy and was not seen in nonsurvivors. In contrast to patients with elevated cortisol levels from either endogenous adrenal production or exogenous treatment, patients with a poor cortisol response to severe illness have also been linked with an increased risk of death.65–67 Overall, these data provide strong evidence that survival is significantly influenced by abnormalities in cortisol production.
Our report has the typical limitations of a retrospective study, including lack of supporting non-glucose laboratory data, and the additional treatment regimens or interventions used. In addition, formal illness severity scoring tools at time of admission, such as the Pediatric Risk of Mortality, were not available although PICU LOS slightly adjusts for this. For this reason, statistical associations with glucose values, medications, LOS, and mortality rates cannot be used to demonstrate causality. Glucose monitoring in our PICU was also not standardized during the study period. Of all potential patients, 372 (23%) were removed from the ultimate analysis. While this potentially affected the estimation of the incidence of hypo- and hyperglycemia, our analysis deliberately focused on patients with multiple glucose values above each particular set cutoff. The very low LOS and mortality rate of the excluded group strengthens the conclusion that these patients, without diabetes mellitus, had a low risk of blood glucose abnormalities.
Our study demonstrates further evidence supporting the association between hypoglycemia, hyperglycemia, and poor outcome in pediatric critical illness. While some of these concepts have been previously reported, this article aims to provide more clinically meaningful prevalence data using multiple glucose values at varying glucose thresholds. Glucocorticoids, and therefore cortisol, have been shown to influence mortality in the critically ill. Our study is the first on this scale to show specific glucocorticoid associations with glucose abnormalities, LOS, and mortality. In the absence of an evidence-based insulin–glucose control protocol, insulin use in our PICU was quite limited. However, its use did correlate with increased LOS and mortality. Overall, these findings illustrate the need for large, prospective trials designed to safely evaluate the use of insulin and glucocorticoids to improve health outcomes in PICU patients.
Acknowledgments
The authors thank Dr. Mark R. Rigby for his critical review of the manuscript and helpful suggestions.
Glossary
Abbreviations
- (CI)
confidence interval
- (CIH)
critical illness hyperglycemia
- (ICD)
International Statistical Classification of Diseases and Related Health Problems
- (ICU)
intensive care unit
- (LOS)
length of stay
- (NICE-SUGAR)
Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation study
- (NOS)
not otherwise specified
- (PICU)
pediatric intensive care unit
References
- 1.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 2.Lewis KS, Kane-Gill SL, Bobek MB, Dasta JF. Intensive insulin therapy for critically ill patients. Ann Pharmacother. 2004;38(7-8):1243–1251. doi: 10.1345/aph.1D211. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 4.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146(1):30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 5.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 6.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 7.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55(1):33–38. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 8.Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann Surg. 1989;210(4):472–473. doi: 10.1097/00000658-198910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75(4):545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- 10.Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46(2):335–343. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Chiaretti A, De Benedictis R, Langer A, Di Rocco C, Bizzarri C, Iannelli A, Polidori G. Prognostic implications of hyperglycaemia in paediatric head injury. Childs Nerv Syst. 1998;14(9):455–459. doi: 10.1007/s003810050259. [DOI] [PubMed] [Google Scholar]
- 12.Chiaretti A, Piastra M, Pulitano S, Pietrini D, De Rosa G, Barbaro R, Di Rocco C. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18(3-4):129–136. doi: 10.1007/s00381-002-0558-3. [DOI] [PubMed] [Google Scholar]
- 13.Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55(6):1035–1038. doi: 10.1097/01.TA.0000031175.96507.48. [DOI] [PubMed] [Google Scholar]
- 14.Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski S, Bayir H, Tyler-Kabara EC, Clark RS, Brown SD, Bell MJ. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2011 doi: 10.1097/PCC.0b013e3182192c30. Epub ahead of print. [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeschke MG, Kraft R, Emdad F, Kulp GA, Williams FN, Herndon DN. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg. 2010;252(3):521–527. doi: 10.1097/SLA.0b013e3181f2774c. discussion 527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 17.Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, Visser FC. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 18.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 19.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22(11):1827–1831. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 20.Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA, Pigula FA, Costello JM. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118(22):2235–2242. doi: 10.1161/CIRCULATIONAHA.108.804286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 23.Van den Berghe G, Bouillon R. Optimal control of glycemia among critically ill patients. JAMA. 2004;291(10):1198–1199. doi: 10.1001/jama.291.10.1198-b. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 25.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Britts RJ, Sakkijah MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 26.De La Rosa Gdel C, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, Saldarriaga NE, Bedoya M, Toro JM, Velásquez JB, Valencia JC, Arango CM, Aleman PH, Vasquez EM, Chavarriaga JC, Yepes A, Pulido W, Cadavid CA. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12(5):R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 28.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 29.Gunst J, Van den Berghe G. Blood glucose control in the intensive care unit: benefits and risks. Semin Dial. 2010;23(2):157–162. doi: 10.1111/j.1525-139X.2010.00702.x. [DOI] [PubMed] [Google Scholar]
- 30.Krinsley JS. Understanding glycemic control in the critically ill: three domains are better than one. Intensive Care Med. 2011;37(3):382–384. doi: 10.1007/s00134-010-2110-3. [DOI] [PubMed] [Google Scholar]
- 31.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preissig CM, Rigby MR, Maher KO. Glycemic control for postoperative pediatric cardiac patients. Pediatr Cardiol. 2009;30(8):1098–1104. doi: 10.1007/s00246-009-9512-4. [DOI] [PubMed] [Google Scholar]
- 33.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37(8):797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 34.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5(4):329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 36.Klein GW, Hojsak JM, Schmeidler J, Rapaport R. Hyperglycemia and outcome in the pediatric intensive care unit. J Pediatr. 2008;153(3):379–384. doi: 10.1016/j.jpeds.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Egi M, Morimatsu H, Toda Y, Matsusaki T, Suzuki S, Shimizu K, Iwasaki T, Takeuchi M, Bellomo R, Morita K. Hyperglycemia and the outcome of pediatric cardiac surgery patients requiring peritoneal dialysis. Int J Artif Organs. 2008;31(4):309–316. doi: 10.1177/039139880803100406. [DOI] [PubMed] [Google Scholar]
- 38.Yung M, Wilkins B, Norton L, Slater A. Glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med. 2008;9(2):147–152. doi: 10.1097/PCC.0b013e3181668c22. [DOI] [PubMed] [Google Scholar]
- 39.Day KM, Haub N, Betts H, Inwald DP. Hyperglycemia is associated with morbidity in critically ill children with meningococcal sepsis. Pediatr Crit Care Med. 2008;9(6):636–640. doi: 10.1097/PCC.0b013e31818d350b. [DOI] [PubMed] [Google Scholar]
- 40.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9(4):361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langouche L, Mesotten D, Vanhorebeek I. Endocrine and metabolic disturbances in critical illness: relation to mechanisms of organ dysfunction and adverse outcome. Verh K Acad Geneeskd Belg. 2010;72(3-4):149–163. [PubMed] [Google Scholar]
- 43.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 44.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 46.Preissig CM, Rigby MR. A disparity between physician attitudes and practice regarding hyperglycemia in pediatric intensive care units in the United States: a survey on actual practice habits. Crit Care. 2010;14(1):R11. doi: 10.1186/cc8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirshberg E, Lacroix J, Sward K, Willson D, Morris AH. Blood glucose control in critically ill adults and children: a survey on stated practice. Chest. 2008;133(6):1328–1335. doi: 10.1378/chest.07-2702. [DOI] [PubMed] [Google Scholar]
- 48.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 49.Arabi YM, Tamim HM, Rishu AH. Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit Care Med. 2009;37(9):2536–2544. doi: 10.1097/CCM.0b013e3181a381ad. [DOI] [PubMed] [Google Scholar]
- 50.Verhoeven JJ, Brand JB, van de Polder MM, Joosten KF. Management of hyperglycemia in the pediatric intensive care unit; implementation of a glucose control protocol. Pediatr Crit Care Med. 2009;10(6):648–652. doi: 10.1097/PCC.0b013e3181ae787b. [DOI] [PubMed] [Google Scholar]
- 51.Preissig CM, Hansen I, Roerig PL, Rigby MR. A protocolized approach to identify and manage hyperglycemia in a pediatric critical care unit. Pediatr Crit Care Med. 2008;9(6):581–588. doi: 10.1097/PCC.0b013e31818d36cb. [DOI] [PubMed] [Google Scholar]
- 52.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 53.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 1997;99(3):414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Raalte DH, Nofrate V, Bunck MC, van Iersel T, Elassaiss Schaap J, Nassander UK, Heine RJ, Mari A, Dokter WH, Diamant M. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162(4):729–735. doi: 10.1530/EJE-09-1034. [DOI] [PubMed] [Google Scholar]
- 55.Van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009;39(2):81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 56.Annane D, Cariou A, Maxime V, Azoulay E, D'Honneur G, Timsit JF, Cohen Y, Wolf M, Fartoukh M, Adrie C, Santré C, Bollaert PE, Mathonet A, Amathieu R, Tabah A, Clec'h C, Mayaux J, Lejeune J, Chevret S. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348. doi: 10.1001/jama.2010.2. [DOI] [PubMed] [Google Scholar]
- 57.Weiss M, Kron M, Hay B, Taenzer M, Radermacher P, Georgieff M. Which variables are associated with blood glucose levels outside the target range in surgical critically ill patients? A retrospective observational study. Patient Saf Surg. 2011;5(1):5. doi: 10.1186/1754-9493-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verhoeven JJ, Hokken-Koelega AC, den Brinker M, Hop WC, van Thiel RJ, Bogers AJ, Helbing WA, Joosten KF. Disturbance of glucose homeostasis after pediatric cardiac surgery. Pediatr Cardiol. 2011;32(2):131–138. doi: 10.1007/s00246-010-9829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141(1):47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 60.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, Laloë V, Muñoz-Sánchez A, Arango M, Hartzenberg B, Khamis H, Yutthakasemsunt S, Komolafe E, Olldashi F, Yadav Y, Murillo-Cabezas F, Shakur H, Edwards P. Effect of intravenous cortico-steroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 61.Sam S, Corbridge TC, Mokhlesi B, Comellas AP, Molitch ME. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf) 2004;60(1):29–35. doi: 10.1111/j.1365-2265.2004.01923.x. [DOI] [PubMed] [Google Scholar]
- 62.Schein RM, Sprung CL, Marcial E, Napolitano L, Chernow B. Plasma cortisol levels in patients with septic shock. Crit Care Med. 1990;18(3):259–263. doi: 10.1097/00003246-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Burchard K. A review of the adrenal cortex and severe inflammation: quest of the “eucorticoid” state. J Trauma. 2001;51(4):800–814. doi: 10.1097/00005373-200110000-00033. [DOI] [PubMed] [Google Scholar]
- 64.Vanhorebeek I, Peeters RP, Vander Perre S, Jans I, Wouters PJ, Skogstrand K, Hansen TK, Bouillon R, Van den Berghe G. Cortisol response to critical illness: effect of intensive insulin therapy. J Clin Endocrinol Metab. 2006;91(10):3803–3813. doi: 10.1210/jc.2005-2089. [DOI] [PubMed] [Google Scholar]
- 65.Sibbald WJ, Short A, Cohen MP, Wilson RF. Variations in adrenocortical responsiveness during severe bacterial infections. Unrecognized adrenocortical insufficiency in severe bacterial infections. Ann Surg. 1977;186(1):29–33. doi: 10.1097/00000658-197707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finlay WE, McKee JI. Serum cortisol levels in severely stressed patients. Lancet. 1982;1(8286):1414–1415. doi: 10.1016/s0140-6736(82)92531-4. [DOI] [PubMed] [Google Scholar]
- 67.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283(8):1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]