Abstract
Objective
The objective is to report a contemporary population-based estimate of hypoglycemia requiring emergency medical services (EMS), its burden on medical resources, and its associated mortality in patients with or without diabetes mellitus (DM, non-DM), which will enable development of prospective strategies that will capture hypoglycemia promptly and provide an integrated approach for prevention of such episodes.
Methods
We retrieved all ambulance calls activated for hypoglycemia in Olmsted County, Minnesota, between January 1, 2003 and December 31, 2009.
Results
A total of 1473 calls were made by 914 people (DM 8%, non-DM 16%, unknown DM status 3%). Mean age was 60 ± 16 years with 49% being female. A higher percentage of calls were made by DM patients (87%) with proportionally fewer calls coming from non-DM patients (11%) (chi-square test, p < .001), and the remaining 2% calls by people with unknown DM status. Emergency room transportation and hospitalization were significantly higher in non-DM patients compared to DM patients (p < .001) and type 2 diabetes mellitus compared to type 1 diabetes mellitus (p < .001). Sulphonylureas alone or in combination with insulin varied during the study period (p = .01). The change in incidence of EMS for hypoglycemia was tracked during this period. However, causality has not been established.
Death occurred in 240 people, 1.2 (interquartile range 0.2–2.7) years after their first event. After adjusting for age, mortality was higher in non-DM patients compared with DM patients (p < .001) but was not different between the two types of DM.
Conclusions
The population burden of EMS requiring hypoglycemia is high in both DM and non-DM patients, and imposes significant burden on medical resources. It is associated with long-term mortality.
Keywords: ambulance, diabetes, emergency room transportation, hypoglycemia
Introduction
Hypoglycemia is a barrier in the achievement of tight glycemic control in diabetes mellitus (DM), making its management difficult and only partially successful. Hypoglycemia may occur in patients without DM (non-DM) because of endocrine, renal, and liver disorders, or after use of alcohol and certain medications.1,2 Hypoglycemia after bariatric surgery has also been reported.3
Often, severe hypoglycemic episodes are treated at home or at the work place by family members, coworkers, and friends without requiring emergency medical services (EMS). Therefore, hypoglycemic episodes requiring EMS and emergency room (ER) visits represent only the “tip of the iceberg”4 but place significant burden on EMS and incur significant expenses from ambulance and hospitalization.5,6
Biological and technological advances in intensive insulin programs [insulin analogs, continuous subcutaneous insulin infusion (CSII) devices, continuous glucose monitoring (CGM) systems, and sensor-augmented insulin pumps] have improved efficacy and safety in randomized clinical trials but their impact on population burden of hypoglycemia requiring EMS remains to be determined.7,8 Clinical trials of intensive glycemic control in patients with type 2 diabetes mellitus (T2DM) have increased concern about severe hypoglycemia and its consequences.9,10 To our knowledge, the utilization of EMS in a population-based study of hypoglycemia has been reported infrequently and no population-based study has reported hypoglycemia in non-DM. The latter is an important question given the increase in bariatric surgery, chronic liver disease (CLD), and end-stage renal disease (ESRD) managed with dialysis on the one hand and the increased risk of severe hypoglycemia on the other. To address these questions, we identified all episodes of hypoglycemia requiring EMS in people with and without DM in a population-based cohort.
In Olmsted County, MN, patients receive subsequent treatment and follow-up primarily at one institution, which facilitates long-term follow-up of this population. We analyzed the incidence, prevalence, risk factors, burden of ER transportation (ERT), and hospitalization, as well as long-term outcomes of EMS requiring hypo-glycemia in Olmsted County. We also report changes in trend of such episodes over the last 7 years and possible reasons for these trend changes.
Methods
After approval from the Institutional Review Board of Mayo Clinic, the database of Gold Cross Ambulance, a corporate, advanced, life-support ground ambulance, was searched for instances of hypoglycemia from January 1, 2003 to December 31, 2009. The population covered was Olmsted County, MN, with an estimate of 141,360 people in 2008.11 Gold Cross Ambulance provides emergency medical care at the scene of medical emergencies and transport to and between medical facilities in Olmsted County and covers more then 95% of ambulance calls in the county.
We used two approaches to ensure that we had identified all patients who required EMS for hypoglycemia. We searched an EMS electronic database maintained for the study period that identified emergency calls for hypoglycemia. Second, we retrieved glucose measure-ments that were less than 3.9 mmol/liter (70 mg/dl) for all ambulance calls during the same period. Gold Cross Ambulance EMS checks plasma glucose with a reflectance glucometer in all subjects with DM and in non-DM patients who present with confusion, seizures, or coma. Subjects above 18 years who authorized the use of their medical records for research were included in the study.
Hypoglycemia, Risk Factors, and Intervention
A structured data abstraction form was used to retrieve the following data: age, gender, type and duration of DM, antihyperglycemic treatment, beta-blocker use, comorbidities contributing to hypoglycemia such as CLD, ESRD, and adrenal insufficiency (AI), target organ compli-cations at baseline, pancreas transplantation, and follow-up including ERT, hospitalization, duration of hospital stay, endocrinology referral services, hypoglycemia recurrence, and major medical events including mortality. Data were also retrieved about ambulance calls made prior to 2003 by the same cohort to confirm hypoglycemic calls as first or recurrent.
Diabetes Comorbidities and Target Organ Complications
Data were gathered using published methods to ascertain DM type and target organ complications.12,13 Diabetes mellitus was classified as type 1 (T1DM) if diagnosed before the age of 35 with ketoacidosis or persistent insulin requirement within 28 days of diagnosis.12 Individuals who did not satisfy these criteria were classified as T2DM. Individuals not on medications for DM and/or with premorbid normal fasting glucose were diagnosed as Non-DM. Chronic liver disease was diagnosed based on the presence of clinically diagnosed cirrhosis, presence of severe cholestatic liver disease with serum bilirubin level more than three times the upper limit of normal for more than 6 months.14 End-stage renal disease was diagnosed on the basis of estimated GFR <15 ml/min or maintenance dialysis.15 Adrenal insufficiency was diagnosed on the basis of a clinical picture consistent with the disorder and confirmatory laboratory results of plasma cortisol and adrenocorticotropic hormone or established therapy for the disorder.16
Mortality
Each patient's vital status (alive/dead) was obtained from Mayo Clinic records and social security death master file. Date of death and underlying cause of mortality were ascertained from medical records and autopsy reports. Patients alive at the end of the study period were treated as censored observations. Hypoglycemia was identified as cause of mortality if the patient died within a few hours of EMS requiring hypoglycemia with no other cause identified. Ascribing death to hypoglycemia is difficult because we have no definitive post-mortem test that serves this purpose.17
Analysis
Continuous measures are reported as mean ± standard deviation (SD) or median and range, while discrete variables are reported as counts and percentages. Differences in baseline factors among T1DM, T2DM, or non-DM patients were tested with Chi-square or analysis of variance methods. Association of mortality with DM status was tested using the log-rank test and a Cox proportional hazards model adjusted for age. An estimated survival function based on the adjusted Cox model was computed using the Breslow estimator of the background hazard function. Cochran-Armitage trend tests were used to test for monotonic (or linear) changes in ERT and hospitalization over time. This analysis was complemented by a logistic regression model with year as a qualitative variable, which alleviated the linearity assumption, i.e., a Type 3 test using 6 degrees of freedom/indicator variables for year.
To estimate the incidence of severe hypoglycemia in the population, standardized rates were estimated using U.S. Census Data (2000), assuming a 1.9% population growth rate. Confidence intervals (CI) were estimated using Poisson approximation and the assumption of independence of hypoglycemic events. Poisson regression was used for testing yearly trends in unadjusted incidence. Chi-square tests were used to test for changes in the distribution of diabetic therapy over the study period. Calculation of the standardized incidence statistics was completed using the IRATE macro that is a part of the Rochester Epidemiology Project.18 Other statistical analyses were performed using JMP 8 and SAS 9 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
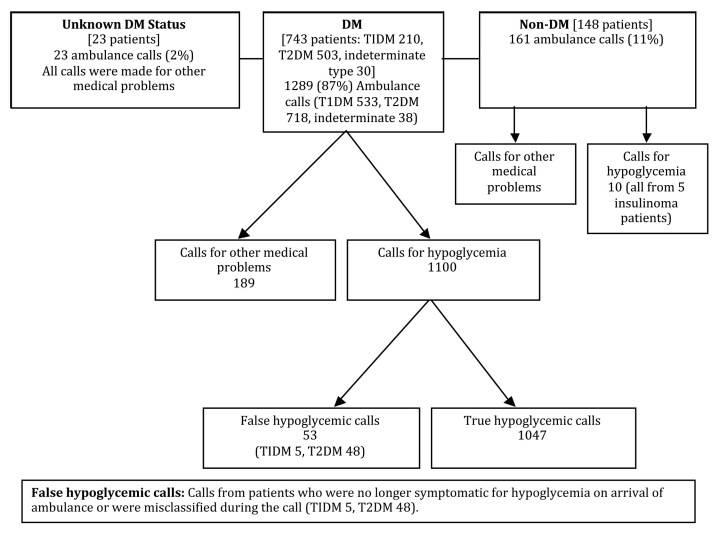
Table 1 shows baseline characteristics of 914 patients who required EMS for hypoglycemia during the study period. Mean age was 60 ± 16 years with 49% being female. A total of 1,473 calls were placed with detailed descriptions given in Figure 1. These calls represent 4.7% of the total 31,540 EMS call made during the study period. Notably, 148 patients (16%) were non-DM who placed 161 (11%) calls. Of the 161 calls from non-DM patients, 10 calls were for hypoglycemia from 5 patients with insulinoma, while the rest were made for other medical problems by patients who also had hypoglycemia. Similarly, in 23 patients with unknown DM status, calls were made for other medical problems. Fifty-three calls from DM patients (T1DM 5, T2DM 48) were classified as false hypoglycemic calls as they had blood glucose more than 70 mg/dl on EMS arrival.
Table 1.
Baseline Demographics for Ambulatory People Who Required Emergency Medical Services for Hypoglycemia in Olmsted County, 2003–2009 (n = 914)
| Totala (n = 914) | T1DM (n = 210) | T2DM (n = 503) | Non-DM (n = 148) | p value | |
|---|---|---|---|---|---|
| Age (SD), years | 60 (15.8) | 47 (13) | 68 (12) | 51 (17) | <.001 |
| Females (%) | 450 (49%) | 97 (46%) | 250 (50%) | 81 (55%) | .28 |
| EMS calls for hypoglycemia distribution, # patients | <.001 | ||||
| 1 Hypoglycemic call | 724 (80%) | 144 (69%) | 393 (78%) | 138 (93%) | |
| 2 Hypoglycemic calls | 100 (10%) | 19 (9%) | 73 (15%) | 7 (5%) | |
| 3 Hypoglycemic calls | 37 (4%) | 15 (7%) | 16 (3%) | 3 (2%) | |
| 4+ Hypoglycemic calls | 53 (6%) | 32 (15%) | 21 (4%) | 0 (0%) | |
| Beta blockers | 324 (35%) | 83 (40%) | 170 (37%) | 51 (35%) | |
| Duration of DM (SD), yearsb | 21 (10.5) | 26 (12) | 16 (9) | NA | |
NA, not applicable.
In the sample of 914 patients, 53 patients were excluded from diabetes classification; 30 patients had diabetes but the type was uncertain, and the remaining 23 participants had limited clinical data to determine diabetes status.
Based on n = 469 with documented diabetes onset date.
Figure 1.
Flow chart presentation of total ambulance calls.
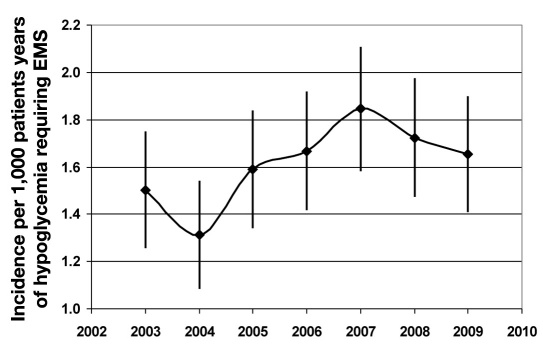
Figure 2 illustrates the annual age- and sex-adjusted incidence rate of hypoglycemia requiring EMS during the study period. Median plasma glucose concentration at first hypoglycemic event was 42 [interquartile range (IQR) 30–59 mg/dl]. The 713 patients with known DM were treated with multiple daily insulin injections (MDI) (45%), CSII (7%), simple insulin program (1–2 insulin injections/day) (23%), combination of oral antihyper-glycemic agents (OAA) and simple insulin program (8%), or OAA (16%). Detailed description about DM treatment among the T1DM and T2DM patients is provided in Table 2. One percent developed hypoglycemia without any antihyperglycemic agent. Insulin-alone use was highest in 2003 (85%) and lowest in 2006 (61%). Sulphonylureas alone or in combination with insulin (SFUI) also varied during the study period (p = .01) being lowest in 2003 (6%) and peaking in 2006–2007 with 20 and 21%, respectively. SFUI use decreased to 17% in 2008 and 12% in 2009. This pattern of use tracked the incidence of EMS for hypoglycemia; however, causality has not been established. Patients were revived with intravenous dextrose (64%), oral glucose (28%), or subcutaneous glucagon (2%). The remaining 86 (6%) patients were not treated because 53 (T1DM 5, T2DM 48) were no longer symptomatic for hypoglycemia or were misclassified during the call. The remaining 33 had plasma glucose between 60 to 70 mg/dl and refused treatment. Hypoglycemia pre-disposing comorbidities (CLD, ESRD, and AI) were higher among DM patients compared to non-DM patients (p < .001).
Figure 2.
Age- and sex-adjusted incidence with 95% CI per 1,000 patient years of hypoglycemia requiring EMS in the Olmsted County population from January 1, 2003 to December 31, 2009. Incidence rate of hypoglycemia requiring EMS increased over the study duration (p = .015) with 2003 and 2004 not differing statistically (p = .3) and incidence rate in 2004 lower than in subsequent years (p < .05 for all comparisons except for 2005, which was p = .08).
Table 2.
Baseline Diabetes Treatment among the T1DM and T2DM Groups
| Total | T1DM | T2DM | |
|---|---|---|---|
| Treatment (n) | 713 | 210 | 503 |
| Simple insulin | 161/713 (23%) | 22/210 (10%) | 139/503 (27%) |
| MDI | 326/713 (45%) | 141/210(67%) | 185/503 (37%) |
| Pump alone | 41/713 (6%) | 37/210 (18%) | 4/503 (1%) |
| Pump with sensors | 7/713 (1%) | 7/210 (3%) | 0/503 (0%) |
| OAA | 116/713 (16%) | 0/210 (0%) | 116/503 (23%) |
| OAA and insulin | 56/713 (8%) | 2/210 (1%) | 54/503 (11%) |
| Off treatment | 6/713 (1%) | 1/ 210 (1%) | 5/503 (1%) |
Patient characteristics differed among T1DM, T2DM, and non-DM. Patients with T2DM were the oldest (p < .001), and the distribution of the number of calls per patient differed statistically between the three groups (p < .001, χ2 with 6 degrees of freedom), with T1DM having the highest percentage of patients with 4 or more calls.
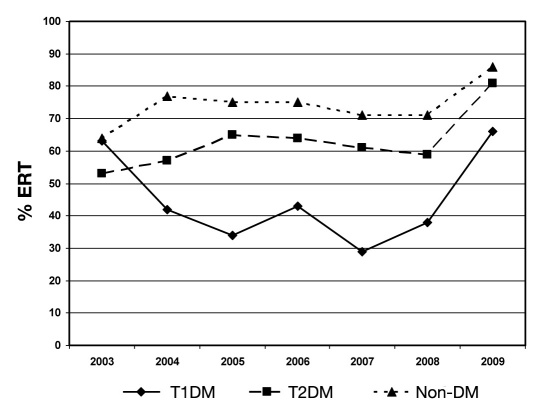
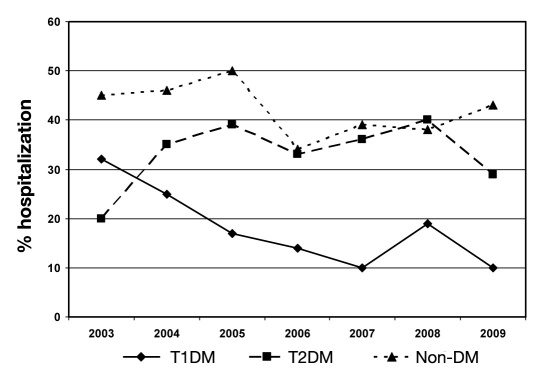
There were also key differences in additional medical service utilization and outcomes by DM classification (Table 3, Figures 3 and 4). Non-DM patients were more likely to require ERT and hospitalization than either DM group (p < .001). However, for hospitalized patients, the length of stay for T1DM tended to be longer than T2DM or non-DM (Tukey-adjusted p values, respectively, p = .05 and .3) and more likely to involve endocrinology consultation (p < .001).
Table 3.
Outcomes of the Olmsted County Cohort Patients Who Needed EMS between 2003–2009
| Total (n = 914) | T1DM (n = 210) | T2DM (n = 503) | Non-DM (n = 148) | p value | |
|---|---|---|---|---|---|
| ERT | 546 (60%) | 108 (51%) | 314 (62%) | 124 (84%) | <.001 |
| Hospitalization | 276 (30%) | 41 (20%) | 166 (33%) | 69 (47%) | <.001 |
| Duration of hospital stay (SD), days | 4.1 (5.8) | 5.9 (10.6) | 3.5 (3.9) | 4.2 (5.2) | .065 |
| Endocrinology consultation | 399 (44%) | 146 (69%) | 239 (47%) | 14 (10%) | <.001 |
| Pancreas transplant | 12 (1%) | 12 (6%) | 0 | NA | <.001 |
| Mortality | 240 (26%) | 33 (16%) | 172 (34%) | 35 (24%) | <.001 |
| Median duration to death in those patients who died, years | 1.2 (IQR 0.2–2.7) | 1.8 (IQR 0.8–4.0) | 1.3 (IQR 0.2–2.7) | 0.1 (IQR 0.01–2.1) | .01 |
| Known causes of mortality | 172/240 (72%) | 25/33 (76%) | 120/172 (70%) | 27/35 (77%) | NA |
| Autopsy confirmation of mortality causes | 121/172 (70%) | 19/25 (76%) | 87/120 (73%) | 15/27 (56%) | NA |
NA, not applicable.
Figure 3.
Yearly trend of ERT. Emergency room transportation rates for the entire sample increased from 59% in 2003 to 79% in 2009 (p = .001 for trend test). The ERT rates for T2DM patients were found to increase monotonically year to year (p = .006, trend test), whereas T1DM patients were found to differ year to year with no linear trend (p = .031 for type 3 analysis; p = .73 for trend). The ERT rates over time were not found to differ for the non-DM patients (p = .23, trend; p = .71, type 3).
Figure 4.
Yearly trend of hospitalization. Hospitalization rates for T1DM patients decreased over the study period (p = .017 for trend test). No changes in hospitalization rates for T2DM or non-DM patients were observed (p = .13 and .095, respectively for type 3 analysis).
Follow-Up and Mortality Analysis
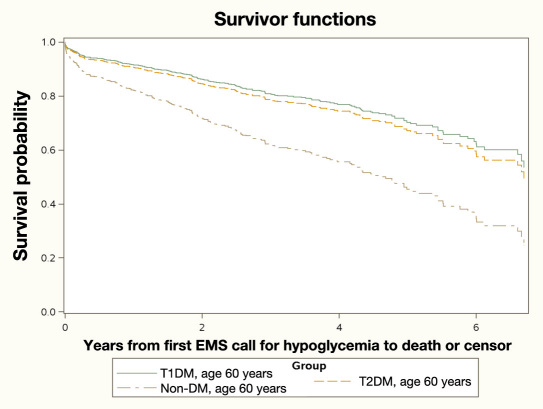
Median follow-up after first episode was 1.9 (IQR 0.6–3.8) years. A total of 240 deaths were observed with 171 events in T2DM patients (Table 3). The survival function examining the relationship of patient classification (T1DM, T2DM, or non-DM) showed an overall difference in the median survival time among the three groups (p < .001, log rank = 22.8 on 2 degrees of freedom). The time from the index hypoglycemic event to mortality, when adjusted for age at first hypoglycemic event, yielded an important observation. The T1DM and T2DM patients had lower hazard for mortality relative to the non-DM patients [hazard ratio (HR) 0.44, 95% CI: 0.28 to 0.73, p = .001; HR = 0.50, 95% CI 0.34 to 0.74, p < .001; respectively) but the hazard between the two DM groups did not differ statistically (p = .6). Figure 5 plots the estimated survival functions for the three groups at the sample-observed mean age of 60 years. Hypoglycemia directly contributed to 3% of overall mortality. All these deaths occurred within a few hours after EMS requiring hypoglycemia. Autopsy report of 2 patients showed encephalopathy consistent with hypoglycemia as cause of death. In the other 4 patients, hypoglycemia was presumed to be cause of death but autopsy was performed in only 1 and revealed no other cause. Major cause of death was respiratory illness, followed by cardiovascular disease and cancer. Most of the deaths in non-DM patients occurred because of respiratory, cerebrovascular, and neurological disorders, while none was directly due to hypoglycemia. In the T2DM cohort, patients died mostly because of respiratory and cardiovascular diseases, while hypoglycemia directly contributed to 3 deaths. In the T1DM cohort, infections, respiratory diseases, and cancers contributed equally to mortality with 3 deaths due to hypoglycemia.
Figure 5.
Estimated survival functions for the three groups at the sample-observed mean age of 60 years. The non-DM cohort had shorter median survival times relative to either the T1DM or T2DM cohorts.
Discussion
Our contemporary study of EMS requiring hypoglycemia confirms that such episodes place significant burden on medical resources and result in long-term morbidity and mortality. Other population-based estimates of hypoglycemia requiring EMS were reported in 2003 or earlier when therapeutic options were limited. Non-DM patients who experienced hypoglycemia constituted 16% of the sample, higher than other reports (6 and 3% respectively).19,20 Both of these studies are more than 7 years old, at a time when therapeutic options were limited. Further, the studies had small sample size and short study periods, with non-DM patients being removed from further analysis.
In our cohort, non-DM patients were at increased risk for mortality compared to DM patients. These patients were more likely to require ERT and hospitalization. This could be due to the more severe nature of the underlying disease. In our study, chronic comorbidities that could cause hypoglycemia were higher in the DM cohort. Because of inability to grade illness severity in outpatients, it is difficult to compare mortality between non-DM and DM cohorts by matching for severity of sickness. Data from inpatient studies have shown association of hypoglycemia with higher mortality but these data were restricted to patients admitted for myocardial ischemia who developed hypoglycemia during the same hospitalization.21 Such evidence is missing for outpatient populations. Thus, hypoglycemia in non-DM outpatient settings may also need to be managed expeditiously with a critical clinical pathway.
The DM group constituted 81% of the cohort, and frequency of calls was 0.75 patient−1·year−1. Emergency medical services were mostly required by T2DM patients (55%), which is expected because of the larger T2DM population. But, the frequency of calls among T2DM patients was lesser than for T1DM patients (0.63 patient−1·year−1 and 0.87 patient−1·year−1, respectively). Frequency of EMS requiring hypoglycemia in the DM, T1DM, and T2DM cohorts was lesser than reports that were published up until 2003; these were limited by small cohort sizes and shorter follow-up periods.5,13 T2DM patients were predominantly on insulin (MDI > simple insulin) followed by SFUI, which is similar to a report from 2003.19 Thus, despite development of insulin analogs, insulin continues to constitute the major risk factor for hypoglycemia in T2DM patients; however, most of the patients still use the traditional MDI and standard injeciton therapy, and data about hypoglycemia with pumps and especially sensor-augmented pumps are in their early years. We also observed hypoglycemia in 6 subjects (5 T2DM, 1 T1DM status postpancreas transplantation) on no antihyper-glycemic therapy. Medical records were complete and reviewed exhaustively to eliminate the possibility of ascertainment error.
Patients with T1DM placed more multiple calls compared with the others. Repeat calls in the DM cohort were comparable to similar reports (23 and 26%, respectively).20,22 One of these studies had more patients on insulin (more details not provided) compared to our cohort, while the other study provided limited details about the type of antihyperglycemic treatment. Also, these studies did not define the size of the T1DM and T2DM groups, which can affect the burden of repeated calls. Repeat calls were higher in the T1DM cohort when compared with a similar report described in 1999 (26%).5 Thus, despite development of new insulin delivery devices, CGM, and sensor-augmented insulin delivery devices, repeated calls may have increased since earlier reports. Patients with T1DM had a longer duration of DM compared with T2DM patients. Association of multiple calls with increasing duration of DM in T1DM patients has been reported.5 An additional risk factor for multiple calls by T1DM patients is a history of prior episodes.13,23 This does not seem to have changed despite the more sophisticated insulin delivery and CGM available since earlier reports. Unfortunately, subjects with repeated severe hypoglycemia have been systematically excluded from prospective studies of CGM and insulin delivery devices.8,24 Prospective studies enrolling such patients may decrease morbidity.
Average age at first episode was comparable between TIDM and non-DM patients, but higher in T2DM patients. No difference was seen in gender distribution of calls among the three cohorts. Earlier data about gender distribution of EMS requiring hypoglycemia is limited among T2DM and non-DM patients but our data in T1DM patients are similar to an earlier report.13
Treatment of hypoglycemia at the scene by paramedics can cut down the cost of ERT and hospitalization and at the same time preserve high patient satisfaction.25 However, in our cohort, more than half of the patients required ERT (60%). Among DM patients, ERT was higher than in earlier reports but later data are not available.13,20 Half of the transported patients were hospitalized for further treatment. Hospitalization among DM patients (30%) was comparable to a study by Leese and colleagues (28%) but higher than other similar reports.6,13,20 ERT rates per ambulance calls for the entire sample increased from 59% in 2003 to 79% in 2009 (Figure 3), which could be because of the more severe nature of hypoglycemic episodes or other factors such as changes in local clinical practice, EMS personnel, reimbursement structure, fear of litigation, or other reasons. Prospective studies with deconstruction of events in real time would be vital to reduce morbidity and mortality in these high-risk populations. Ginde and colleagues6 observed no change in ERT per 1,000 DM patients with or total ER visits between 1993–2005 without involving Non-DM patients but did not report data about EMS. Further, this large study did not report morbidity in different types of DM patients or details of DM therapy, and therefore has limited value regarding planning of interventions to change trends and improve outcomes.
A large number of patients (26%) died during the study period with respiratory illnesses as the main cause of mortality followed by cardiovascular diseases. It is difficult to establish the cause and effect association from the temporal relationship of hypoglycemia and death. Hypoglycemia-associated mortality in our cohort (3%) was higher than a similar report in 2003.19 Fifteen percent of patients died during the same hospitalization with the main reasons being respiratory illness and sepsis. Causes of death after EMS requiring hypoglycemia have not been reported in a population-based study.
This is the first population-based study of EMS requiring hypoglycemia with a detailed description of incidence, burden on medical resources utilization, and mortality among DM and non-DM patients. Non-DM patients have been excluded from the earlier studies; however, these patients may need to be managed with a critical clinical pathway due to worse outcomes compared to DM patients. Also, our study provides updated information about the population burden of EMS requiring hypo-glycemia. Limitations of our study include slight inflation of hypoglycemia incidence due to a large transient population visiting the Mayo Clinic who are at risk for hypo-glycemia. Besides severity of hypoglycemia, ambulance calls for a patient could be driven by reasons such as the caller's familiarity with hypoglycemia, availability of glucagon, past frightening experiences with severe hypoglycemia, and emotional reactions to events such as vehicular accidents, etc.
In conclusion, hypoglycemia requiring EMS places significant burden on medical resources and is associated with significant long-term morbidity and mortality. Hypoglycemia in non-DM patients was more severe requiring higher ERT and hospitalization and had higher mortality compared to DM patients. Prospective strategies need to be developed and evaluated that capture hypoglycemia promptly and include an integrated approach for prevention of such episodes, resulting in alleviation of patient discomfort, decreased burden on hospital services, improved long-term outcomes, and decreased morbidity and mortality.
Acknowledgments
We thank Gold Cross Ambulance for help with data retrieval and Ms. Tina Miller for help with our patients and their families and formatting the manuscript.
Glossary
Abbreviations
- (AI)
adrenal insufficiency
- (CGM)
continuous glucose monitoring
- (CI)
confidence interval
- (CLD)
chronic liver disease
- (CSII)
continuous subcutaneous insulin delivery
- (DM)
diabetes mellitus
- (EMS)
emergency medical services
- (ER)
emergency room
- (ERT)
emergency room transportation
- (ESRD)
end-stage renal disease
- (HR)
hazard ratio
- (IQR)
interquartile range
- (MDI)
multiple daily insulin injection
- (non-DM)
with no diabetes mellitus
- (OAA)
oral antihyperglycemic agents
- (SD)
standard deviation
- (SFUI)
sulphonylureas alone or in combination with insulin
- (TIDM)
type 1 diabetes mellitus
- (T2DM)
type 1 diabetes mellitus
Funding
This study was supported by internal research funding from the Mayo Foundation.
References
- 1.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 2.Murad MH, Coto-Yglesias F, Wang AT, Sheidaee N, Mullan RJ, Elamin MB, Erwin PJ, Montori VM. Clinical review: drug-induced hypoglycemia: a systematic review. J Clin Endocrinol Metab. 2009;94(3):741–745. doi: 10.1210/jc.2008-1416. [DOI] [PubMed] [Google Scholar]
- 3.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 4.Potter J, Clarke P, Gale EA, Dave SH, Tattersall RB. Insulin-induced hypoglycaemia in an accident and emergency department: the tip of an iceberg? Br Med J (Clin Res Ed). 1982;285(6349):1180–1182. doi: 10.1136/bmj.285.6349.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels A, White M, Stander I, Crone D. Ambulance visits for severe hypoglycaemia in insulin-treated diabetes. N Z Med J. 1999;112(1090):225–228. [PubMed] [Google Scholar]
- 6.Ginde AA, Espinola JA, Camargo CA., Jr Trends and disparities in U.S. emergency department visits for hypoglycemia, 1993–2005. Diabetes Care. 2008;31(3):511–513. doi: 10.2337/dc07-1790. [DOI] [PubMed] [Google Scholar]
- 7.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 8.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 9.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT, Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau. State & County QuickFacts: Olmstead County, Minnesota. [updated 2011 Oct 27, cited 2009 Dec 24]. Available from: http://quickfacts.census.gov/qfd/states/27/27109.htm.
- 12.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 13.Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD DARTS/MEMO Collaboration. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 14.Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(Suppl 1):i1–i9. doi: 10.1136/gut.50.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. Calculators for health care professionals. [cited 2010 Mar]. Available from: http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm.
- 16.Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059–1067. doi: 10.1210/jc.2009-0032. [DOI] [PubMed] [Google Scholar]
- 17.Frier B, Fisher M. Hypoglycemia and diabetes: clinical and physiological aspects. In: Tattersall R, Gale E, editors. Mortality. Vol. 1. London: Edward Arnold Publications; 1993. pp. 190–197. [Google Scholar]
- 18.Mayo Clinic. Rochester Epidemiology Project (REP) Accessible from: http://hsrwww.mayo.edu/epi/rep/rep_project_manual.html. Accessed on November 6, 2010.
- 19.Holstein A, Plaschke A, Vogel MY, Egberts EH. Prehospital management of diabetic emergencies–a population-based intervention study. Acta Anaesthesiol Scand. 2003;47(5):610–615. doi: 10.1034/j.1399-6576.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 20.Socransky SJ, Pirrallo RG, Rubin JM. Out-of-hospital treatment of hypoglycemia: refusal of transport and patient outcome. Acad Emerg Med. 1998;5(11):1080–1085. doi: 10.1111/j.1553-2712.1998.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 21.Kosiborod M, Inzucchi SE, Goyal A, Krumholz HM, Masoudi FA, Xiao L, Spertus JA. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301(15):1556–1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 22.Cain E, Ackroyd-Stolarz S, Alexiadis P, Murray D. Prehospital hypoglycemia: the safety of not transporting treated patients. Prehosp Emerg Care. 2003;7(4):458–465. doi: 10.1080/312703002193. [DOI] [PubMed] [Google Scholar]
- 23.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 24.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2009;94(3):729–740. doi: 10.1210/jc.2008-1415. [DOI] [PubMed] [Google Scholar]
- 25.Carter AJ, Keane PS, Dreyer JF. Transport refusal by hypoglycemic patients after on-scene intravenous dextrose. Acad Emerg Med. 2002;9(8):855–857. doi: 10.1111/j.1553-2712.2002.tb02179.x. [DOI] [PubMed] [Google Scholar]