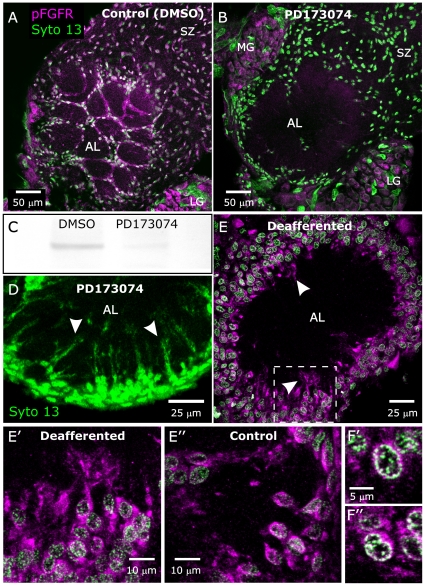
Figure 6. Blocking activation of the FGFR blocks migration of neuropil glia.
A,B: Animals injected at stage 4 with DMSO or DMSO + PD173074 and examined at stage 7. A: Control animal injected with vehicle (DMSO) and labeled with the anti-pFGFR antibody (magenta) and Syto 13 (green) to show cell nuclei. Neuropil glial cells have migrated to surround glomeruli as in untreated animals. A more anterior view than those used in Fig. 2 was chosen to better illustrate the intense labeling of NP glial processes surrounding glomeruli. B: Animal injected with DMSO containing 0.5 mg PD173074. Labeling for activated FGFRs on glial cells is absent, and most neuropil-associated (NP) glia have failed to migrate to surround glomeruli. Sorting zone (SZ) glia have migrated normally despite their lack of labeling for activated FGFRs. C: A western blot of control and PD173074-treated antennal-lobe tissue from which neuronal cell bodies had been removed demonstrates a nearly complete absence of labeling for pFGFRs for the PD173074-treated lobes. D: Another animal treated with 0.5 mg PD173074 (beginning at stage 3), dissected at stage 7, and labeled with Syto 13. At increased gain, some NP glial processes can be seen to have extended into the neuropil (arrowheads) despite the absence of cell-body migration. E: A stage-6 antennal lobe from an animal chronically deprived of ORN innervation on one side (antennal anlagen removed at stage 1.) Although lack of ORN innervation resulted in lack of glial migration, glial cells did exhibit activated FGFRs (magenta, arrowheads). E′: Enlarged section from boxed area of panel E better illustrates the labeling of glial processes. E″: Opposite lobe, which was not deprived of ORN input, appears to have the same intensity of pFGFR labeling. F′,F″: Individual cells of deafferented (F′) and control (F″) lobes both display colocalization of pFGFR and DNA labels. LG, MG = lateral and medial group of AL neuron cell bodies. Projection depths = 10 µm in A, B, D, E, E′. F′, F″ are single optical sections (40× objective).