FIGURE 6.
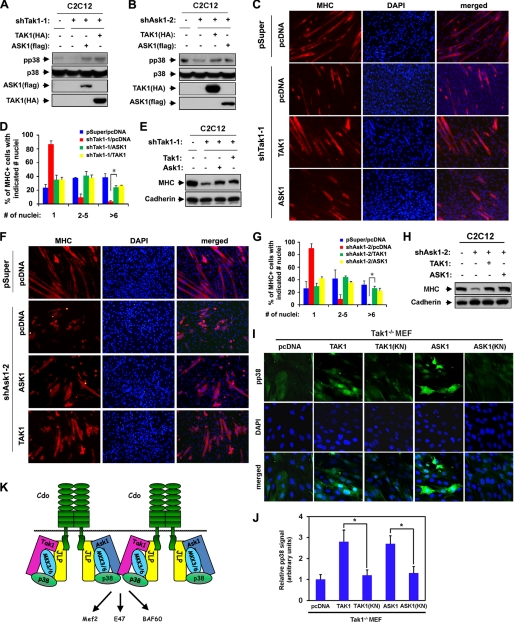
Overexpression of ASK1 or TAK1 can reactivate p38MAPK in TAK1- or ASK1-depleted cells and restore myogenic differentiation. A, C2C12/pSuper and C2C12/shTak1-1 cells were transfected with TAK1(HA), ASK1(FLAG), or control (−) expression vectors as indicated, and the lysates were subjected to Western blotting with antibodies to pp38, p38, FLAG (for ASK1), or HA (for TAK1). B, C2C12/pSuper and C2C12/shAsk2-1 cells were transfected with TAK1(HA), ASK1(FLAG), or control (−) expression vectors as indicated, and the lysates were subjected to Western blotting with antibodies to pp38, p38, FLAG, or HA. C, photomicrographs of C2C12/pSuper and C2C12/shTak1-1 cells transfected with TAK1(HA), ASK1(FLAG), or pcDNA expression vectors cultured in DM for 2 days, fixed, and immunostained with an antibody to MHC (red). Nuclei were visualized by staining with DAPI (blue). D, quantification of myotube formation by the cell lines shown in C. Values represent the means of triplicate determinations ± 1 S.D. The experiments were repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. E, lysates of cell lines shown in C were analyzed for myoblast differentiation by immunoblotting with antibodies to MHC and to cadherin as the loading control. F, photomicrographs of C2C12/pSuper and C2C12/shAsk2-1 cells transfected with TAK1(HA), ASK1(FLAG), or pcDNA expression vectors cultured in DM for 2 days, fixed, and immunostained with an antibody to MHC (red). Nuclei were visualized by staining with DAPI (blue). G, quantification of myotube formation by the cell lines shown in F. Values represent the means of triplicate determinations ± 1 S.D. The experiments were repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. H, lysates of cell lines shown in F were analyzed for myoblast differentiation by immunoblotting with antibodies to MHC and to cadherin as a loading control. I, Tak1−/− MEF cells were transfected sequentially with MyoD expression vectors and expression vectors for ASK1, ASK1(KN), TAK1, TAK1(KN), or the control. Cells were induced to differentiate for 48 h in DM, fixed, and stained with antibodies to pp38MAPK (green). Cell nuclei were visualized by staining with DAPI (blue). J, quantification of cultures shown in I. The intensity of the immunofluorescent pp38 signals was quantified, with the values obtained from control vector-transfected cultures set to 1.0. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. K, schematic diagram summarizing the role of ASK1 and TAK1 as MAP3Ks in Cdo-initiated p38MAPK activation.