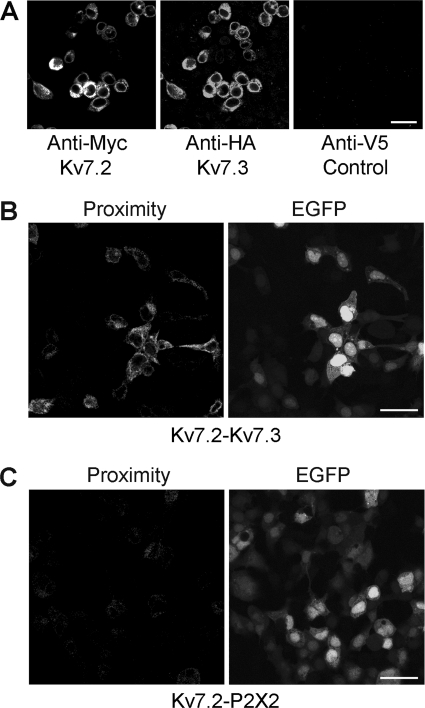
FIGURE 1.
Expression and interaction between Myc-Kv7.2 and 2xHA-Kv7.3. A, immunofluorescence detection of epitope-tagged proteins in transiently transfected tsA 201 cells. Cotransfected cells were fixed, permeabilized, and incubated with appropriate anti-tag antibodies, as indicated, followed by fluorophore-conjugated secondary antibodies. Cells were imaged by confocal laser scanning microscopy. B, in situ proximity ligation assay for Kv7.2/Kv7.3 interaction. Cells were cotransfected with DNA encoding Myc-Kv7.2 and 2xHA-Kv7.3. Transfections also included pEGFP to identify transfected cells. Cells were fixed, permeabilized, and incubated with primary antibodies (rabbit polyclonal anti-Myc and mouse monoclonal anti-HA) followed by anti-mouse (+) and anti-rabbit (-) proximity ligation secondary antibodies. The proximity ligation assay was then carried out, and treated cells were imaged by confocal laser scanning microscopy. The left panel shows the proximity signal, and the right panel shows the EGFP signal. C, control experiment in which cells were transfected with Myc-Kv7.2 plus His6-P2X2 receptor. In this case, primary antibodies were rabbit polyclonal anti-Myc and mouse monoclonal anti-His6. Transfected cells are indicated by the EGFP signal (right panel). There is a considerable reduction in the proximity ligation signal (left panel). Scale bars = 20 μm.