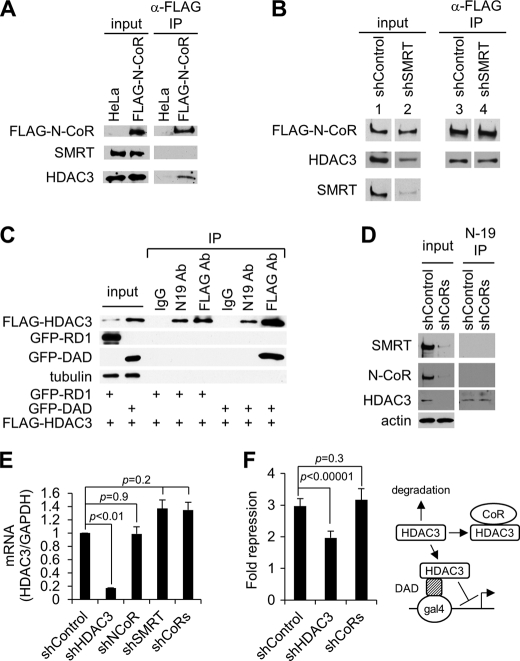
FIGURE 4.
Effect of SMRT knockdown on the formation of N-CoR-HDAC3 complex and effect of corepressor depletion on the expression of free HDAC3. A, Western blot analysis of indicated proteins in the input and anti-FLAG M2-agarose immunoprecipitates obtained from nuclear extract of HeLa cells stably expressing FLAG-N-CoR. B, Western blot analysis of indicated proteins in the input and anti-FLAG M2-agarose immunoprecipitates obtained from cell extracts of the FLAG-N-CoR cell line transduced with either control or SMRT shRNA. Lanes 1–4 were from non-adjacent lanes on the same gel. C, Western blot analysis of indicated proteins in the input and immunoprecipitates from cells co-transfected with FLAG-HDAC3 along with GFP-RD1 or GFP-DAD. Immunoprecipitation was carried out using either control IgG, N-19 anti-HDAC3, or anti-FLAG antibodies (Ab). FLAG-HDAC3 was detected by using the anti-FLAG antibody. D, Western blot analysis of indicated proteins in the input as well as the N19 anti-HDAC3 immunoprecipitates obtained from HeLa cells depleted of both N-CoR and SMRT by shRNAs (shCoRs). E, quantitative real-time PCR showing that knockdown of N-CoR and SMRT corepressors does not significantly affect the mRNA level of HDAC3 in HeLa cells. HDAC3 levels were normalized to the level of GAPDH under each treatment condition, and the relative expression of HDAC3 was set as 1 in control shRNA-transduced cells. F, luciferase reporter assays performed in 293T cells co-transfected with a Gal4-UAS-SV40 luciferase reporter along with Gal4-DAD fusion protein as well as the different shRNA constructs, as indicated. The fold repression is relative to the luciferase activities observed in cells transfected with Gal4 DNA-binding domain along with each shRNA construct. Right panel, schematic representation showing the different HDAC3 pathways in this assay system. Only free HDAC3 can be recruited by Gal4-DAD to repress transcription from the transfected reporter.