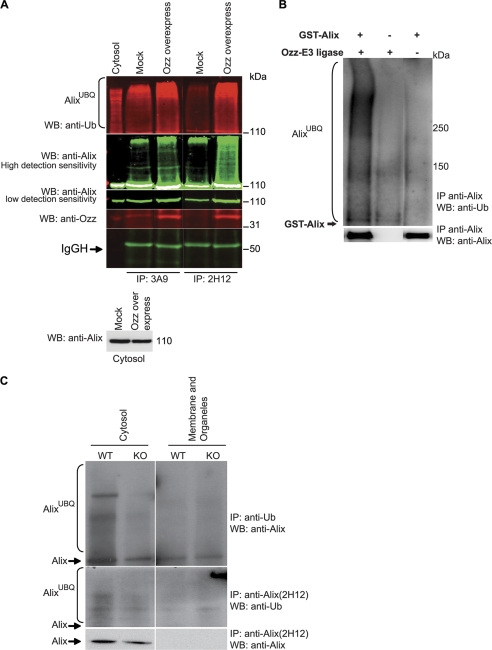
FIGURE 3.
Ozz-E3 complex ubiquitinates Alix in vitro and in vivo. A, the ubiquitination of Alix was assessed in the cytosolic fractions of C2C12 transfected with an empty vector (Mock) or a vector containing Ozz cDNA (Ozz overexpressing), at D3 of differentiation. Proteins were immunoprecipitated (IP) with two anti-Alix antibodies (clone 3A9 and 2H12) and immunoblotted with the indicated antibodies. The level of Alix in the cytosolic fraction is also reported. Band intensities were acquired using an Odyssey Infrared Imaging System (LI-COR), at low or high detection sensitivity. WB, Western blot. B, in vitro ubiquitination of GST-tagged Alix was performed in presence or absence of the reconstituted Ozz-E3 complex and GST-Alix. Ubiquitinated Alix was immunoprecipitated with anti-Alix and detected on immunoblot probed with anti-ubiquitin. C, Alix level and the extent of Alix ubiquitination was compared in subcellular fractions (cytosol and membrane/organelle) of Ozz+/+ (WT) and Ozz−/− (KO) primary myotubes. Anti-ubiquitin immunoprecipitated more ubiquitinated Alix in the cytosolic fraction of the wild-type myotubes at D3 of differentiation than in the Ozz null myotubes. Similarly, the amount of native Alix immunoprecipitated with the anti-Alix clone 2H12 was significantly more in the cytosolic fraction of the wild-type than in the Ozz null myotubes.