Abstract
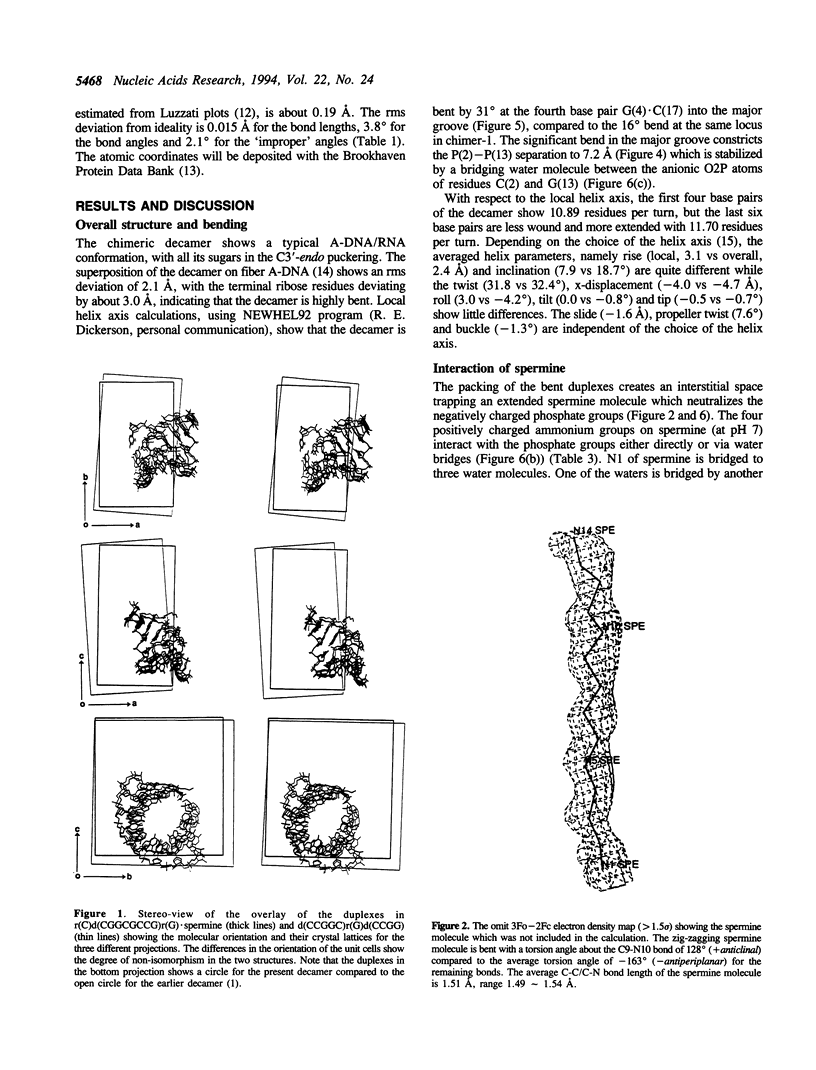
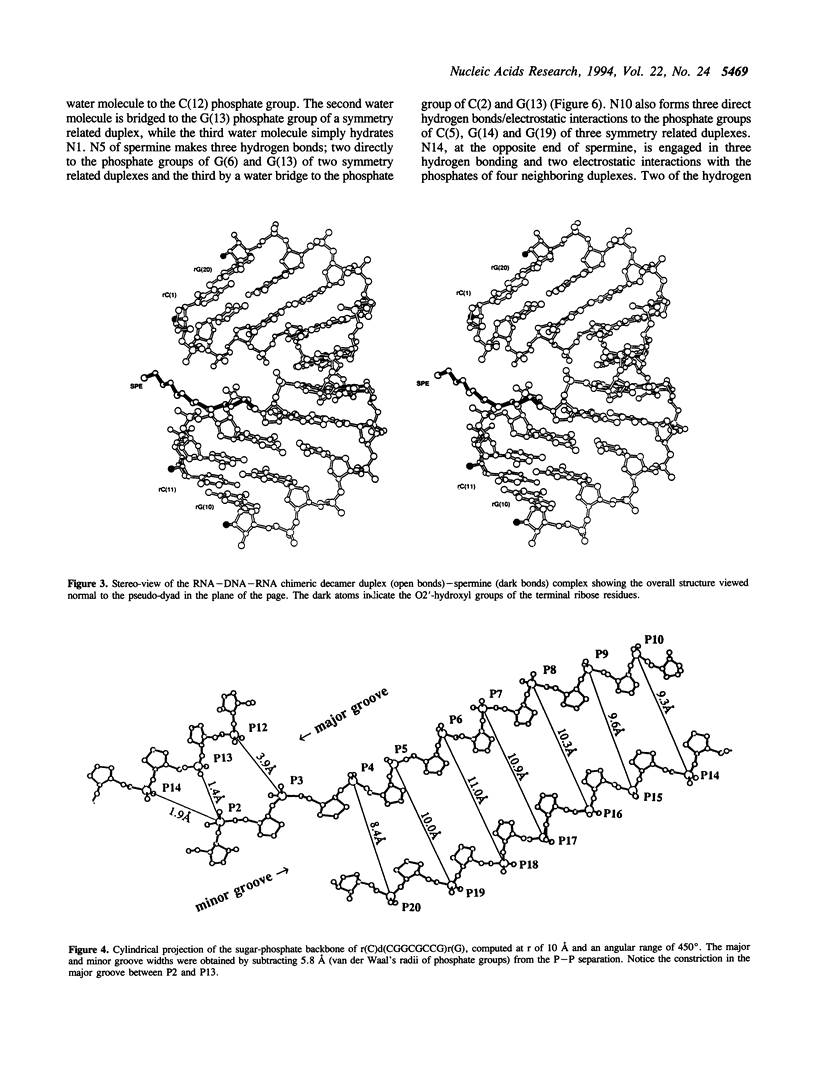
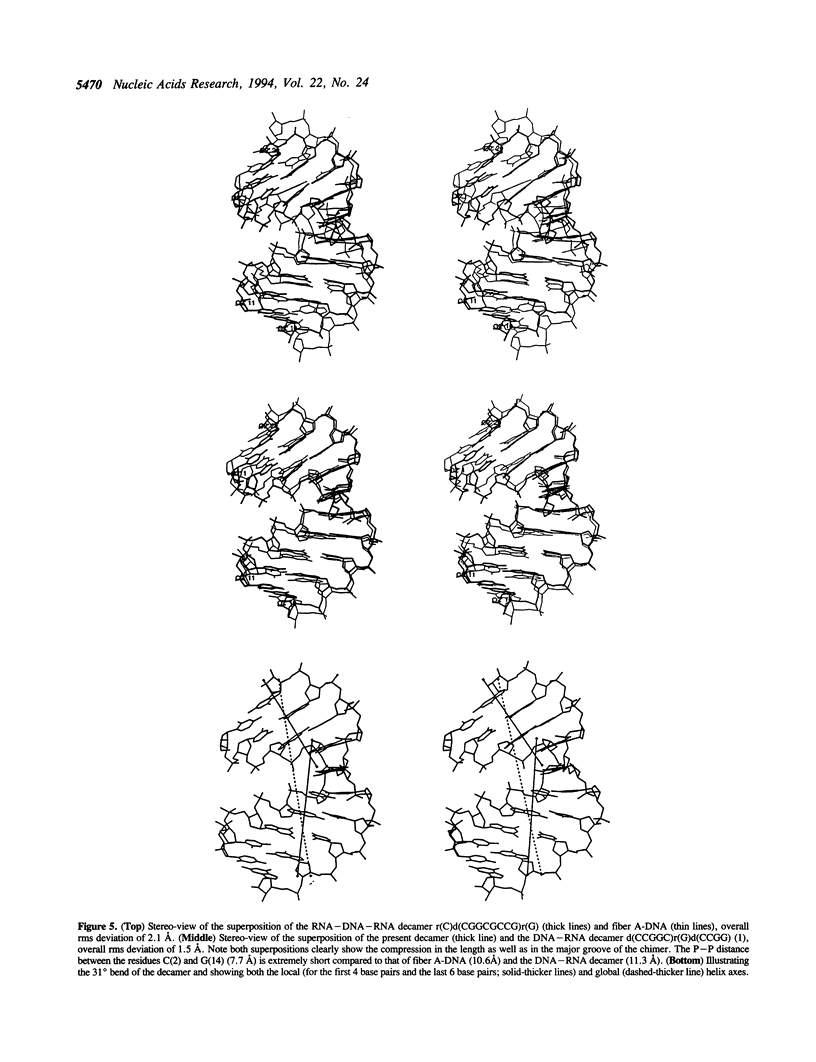
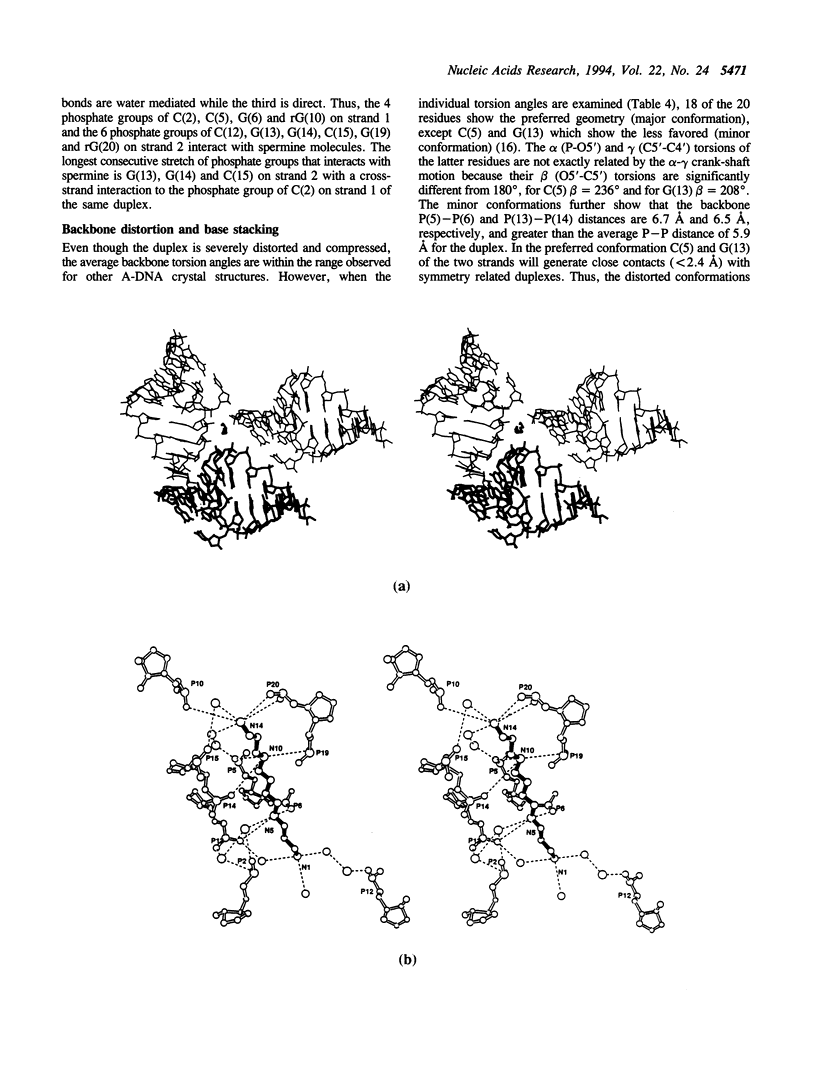
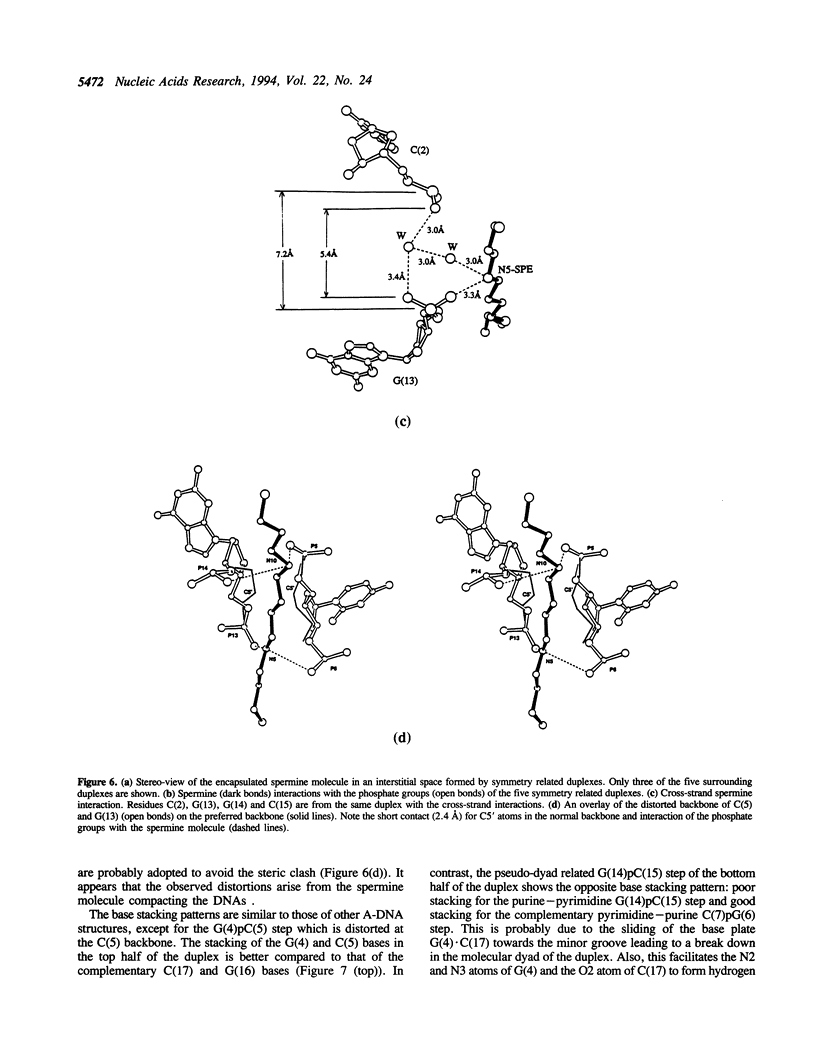
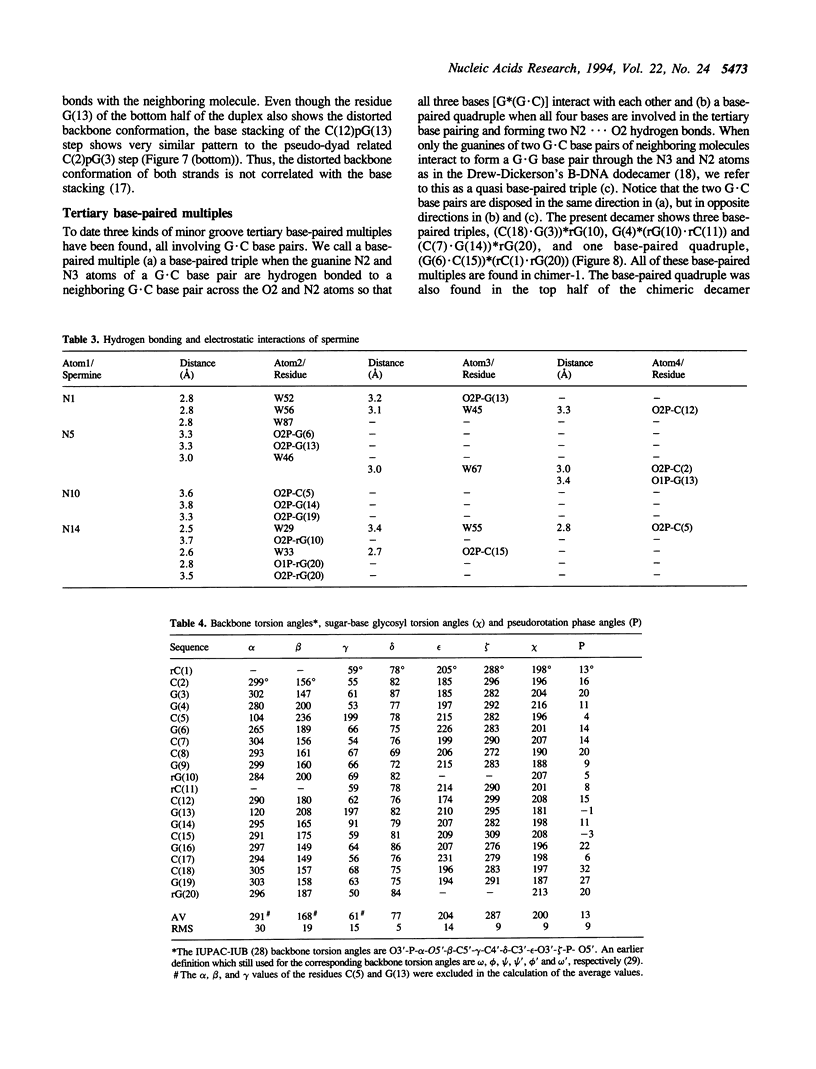
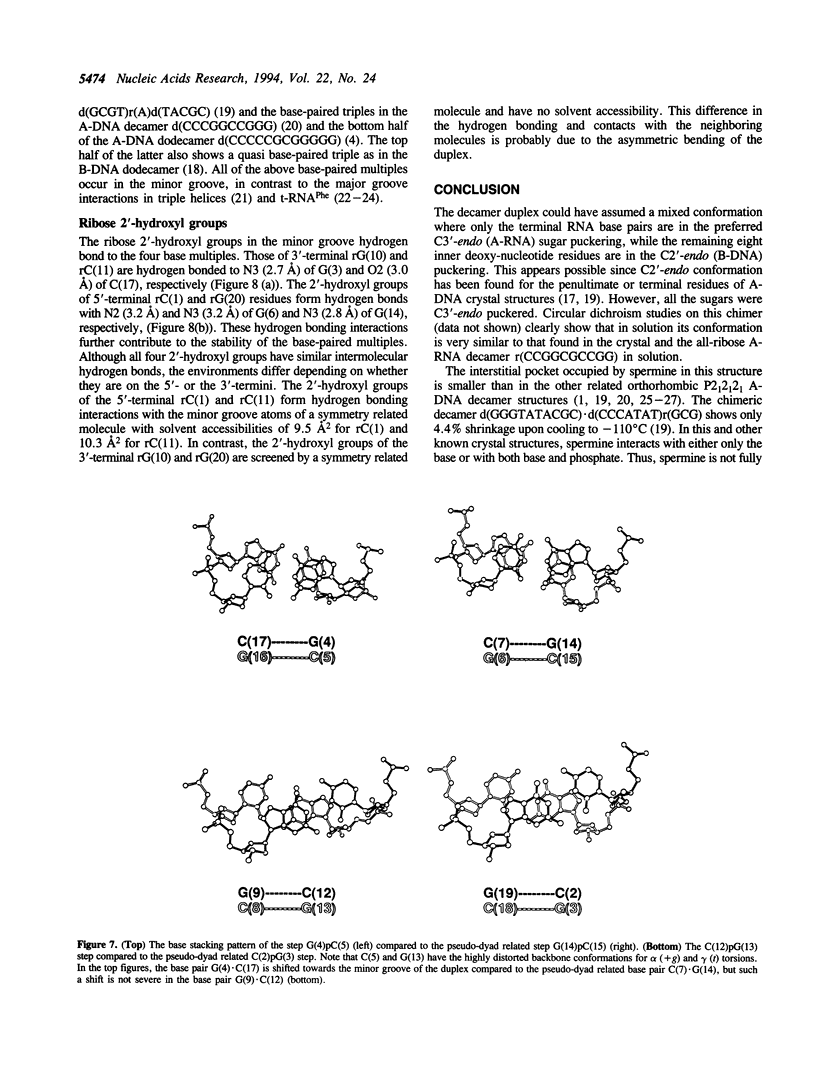
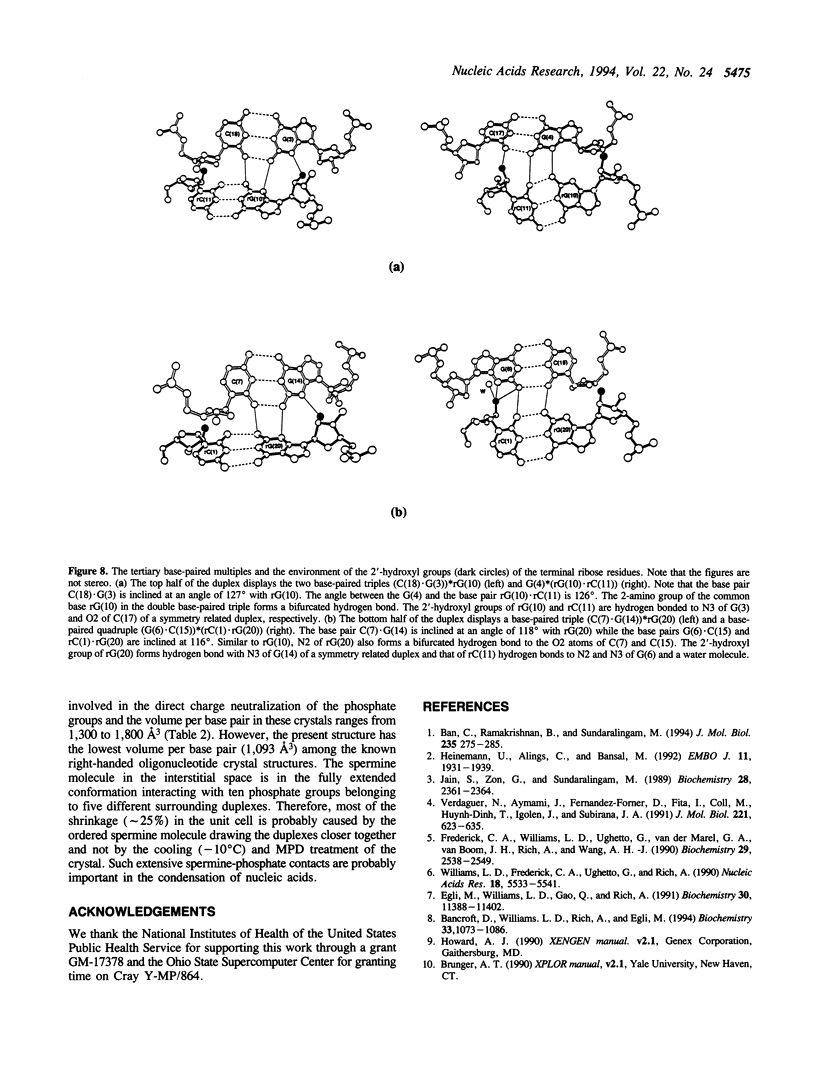
The crystal structure of the self-complementary chimeric decamer duplex r(C)d(CGGCGCCG)r(G), with RNA base pairs at both termini, has been solved at 1.9 A resolution by the molecular replacement method and refined to an R value of 0.145 for 2,314 reflections. The C3'-endo sugar puckers of the terminal riboses apparently drive the entire chimeric duplex into an A-DNA conformation, in contrast to the B-DNA conformation adopted by the all-deoxy decamer of the same sequence. Five symmetry related duplexes encapsulate a spermine molecule which interacts with ten phosphate groups, both directly and through water molecules to form multiple ionic and hydrogen bonding interactions. The spermine interaction severely bends the duplexes by 31 degrees into the major groove at the fourth base pair G(4).C(17), jolts it and slides the 'base plate' into the minor groove. This base pair, together with the adjacent base pair in the top half and the corresponding pseudo two-fold related base pairs in the bottom half, form four minor groove base-paired multiples with the terminal base pairs of two neighboring duplexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban C., Ramakrishnan B., Sundaralingam M. A single 2'-hydroxyl group converts B-DNA to A-DNA. Crystal structure of the DNA-RNA chimeric decamer duplex d(CCGGC)r(G)d(CCGG) with a novel intermolecular G-C base-paired quadruplet. J Mol Biol. 1994 Feb 11;236(1):275–285. doi: 10.1006/jmbi.1994.1134. [DOI] [PubMed] [Google Scholar]
- Bancroft D., Williams L. D., Rich A., Egli M. The low-temperature crystal structure of the pure-spermine form of Z-DNA reveals binding of a spermine molecule in the minor groove. Biochemistry. 1994 Feb 8;33(5):1073–1086. doi: 10.1021/bi00171a005. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bingman C. A., Zon G., Sundaralingam M. Crystal and molecular structure of the A-DNA dodecamer d(CCGTACGTACGG). Choice of fragment helical axis. J Mol Biol. 1992 Oct 5;227(3):738–756. doi: 10.1016/0022-2836(92)90221-5. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran R., Wang M., He R. G., Puigjaner L. C., Byler M. A., Millane R. P., Arnott S. A re-examination of the crystal structure of A-DNA using fiber diffraction data. J Biomol Struct Dyn. 1989 Jun;6(6):1189–1202. doi: 10.1080/07391102.1989.10506544. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Wing R. M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M., Usman N., Rich A. Conformational influence of the ribose 2'-hydroxyl group: crystal structures of DNA-RNA chimeric duplexes. Biochemistry. 1993 Apr 6;32(13):3221–3237. [PubMed] [Google Scholar]
- Egli M., Usman N., Zhang S. G., Rich A. Crystal structure of an Okazaki fragment at 2-A resolution. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):534–538. doi: 10.1073/pnas.89.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M., Williams L. D., Gao Q., Rich A. Structure of the pure-spermine form of Z-DNA (magnesium free) at 1-A resolution. Biochemistry. 1991 Dec 3;30(48):11388–11402. doi: 10.1021/bi00112a005. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Williams L. D., Ughetto G., van der Marel G. A., van Boom J. H., Rich A., Wang A. H. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990 Mar 13;29(10):2538–2549. [PubMed] [Google Scholar]
- Heinemann U., Alings C., Bansal M. Double helix conformation, groove dimensions and ligand binding potential of a G/C stretch in B-DNA. EMBO J. 1992 May;11(5):1931–1939. doi: 10.1002/j.1460-2075.1992.tb05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IITAKA Y., HUSE Y. THE CRYSTAL STRUCTURE OF SPERMINE PHOSPHATE HEXAHYDRATE. Acta Crystallogr. 1965 Jan 10;18:110–121. doi: 10.1107/s0365110x65000191. [DOI] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Base only binding of spermine in the deep groove of the A-DNA octamer d(GTGTACAC). Biochemistry. 1989 Mar 21;28(6):2360–2364. doi: 10.1021/bi00432a002. [DOI] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Hexagonal crystal structure of the A-DNA octamer d(GTGTACAC) and its comparison with the tetragonal structure: correlated variations in helical parameters. Biochemistry. 1991 Apr 9;30(14):3567–3576. doi: 10.1021/bi00228a030. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Klug A., Ladner J., Robertus J. D. The structural geometry of co-ordinated base changes in transfer RNA. J Mol Biol. 1974 Nov 5;89(3):511–516. doi: 10.1016/0022-2836(74)90480-x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan B., Sundaralingam M. Evidence for crystal environment dominating base sequence effects on DNA conformation: crystal structures of the orthorhombic and hexagonal polymorphs of the A-DNA decamer d(GCGGGCCCGC) and comparison with their isomorphous crystal structures. Biochemistry. 1993 Oct 26;32(42):11458–11468. doi: 10.1021/bi00093a025. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan B., Sundaralingam M. High resolution crystal structure of the A-DNA decamer d(CCCGGCCGGG). Novel intermolecular base-paired G*(G.C) triplets. J Mol Biol. 1993 May 20;231(2):431–444. doi: 10.1006/jmbi.1993.1292. [DOI] [PubMed] [Google Scholar]
- Verdaguer N., Aymami J., Fernández-Forner D., Fita I., Coll M., Huynh-Dinh T., Igolen J., Subirana J. A. Molecular structure of a complete turn of A-DNA. J Mol Biol. 1991 Sep 20;221(2):623–635. doi: 10.1016/0022-2836(91)80077-8. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., van der Marel G. A., van Boeckel S. A., Rich A. Molecular structure of r(GCG)d(TATACGC): a DNA--RNA hybrid helix joined to double helical DNA. Nature. 1982 Oct 14;299(5884):601–604. doi: 10.1038/299601a0. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Frederick C. A., Ughetto G., Rich A. Ternary interactions of spermine with DNA: 4'-epiadriamycin and other DNA: anthracycline complexes. Nucleic Acids Res. 1990 Sep 25;18(18):5533–5541. doi: 10.1093/nar/18.18.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]



