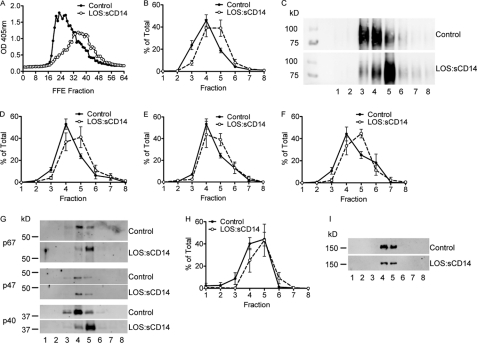
FIGURE 6.
Endotoxin priming of PMN elicits an alteration in the properties of the light membrane subfractions. A, alkaline phosphatase activity assay performed on the FFE subfractions from control and endotoxin-primed, 10 ng/ml LOS:sCD14. PMN demonstrates a shift in the fractions displaying alkaline phosphatase activity post-Triton X-100 permeabilization, representative activity assay, from n = 5 individual experiments. FFE subfractions were pooled into groups of 8 for further biochemical analysis. B–C, gp91phox protein was detected primarily in pooled subfractions 3, 4, and 5 from control PMNs, but shifted to fractions 4, 5, and 6 following endotoxin priming. B, compiled data from n = 5 separate FFE runs. C, representative immunoblot for gp91phox. D–F, cytosolic subunits of the oxidase were also associated with light membrane pooled subfractions isolated by FFE. p40phox (D) and p67phox (F) displayed similar shifts by immunoblotting as seen with gp91phox, whereas p47phox (E) had only a minor shift following priming, n = 5. G, representative immunoblot for control versus LOS:sCD14 primed PMNs for cytosolic subunits of Nox2. H–I, there was no shift in the clathrin heavy chain protein content of FFE subfractions following endotoxin priming, n = 5.