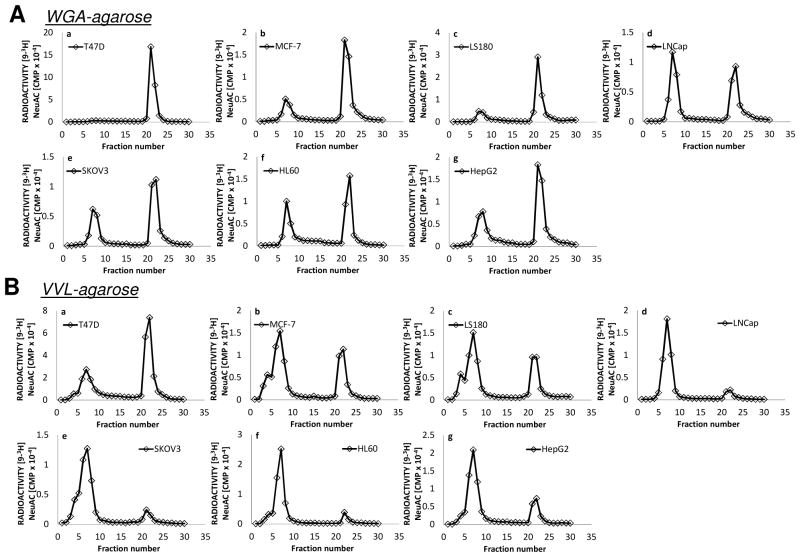
Fig 1. Lectin affinity chromatography of intact proteins.
Lectin-agarose affinity chromatography of [9-3H] sialylated glycoproteins of lysates from human cancer cell lines a: T47D; b: MCF-7; c: LS180; d: LNCaP; e: SKOV3; f: HL60; g: HepG2. A column of 7ml bed volume of WGA-agarose (top half of figure, A) or VVL-agarose (bottom half of figure, B) was employed using 10mM Hepes pH7.5 containing 0.1mM CaCl2, 0.01mM MnCl2 and 0.1% NaN3 as the running buffer. An aliquot (either 50 or 100 μL) of [9-3H] sialyl dialyzed cell extract preparation was diluted to 1 mL with the running buffer and applied to the affinity column. Fractions of 1.0ml were collected. After fraction 15, the bound material was eluted with 0.5M GlcNAc or 0.2M GalNAc in the same buffer.