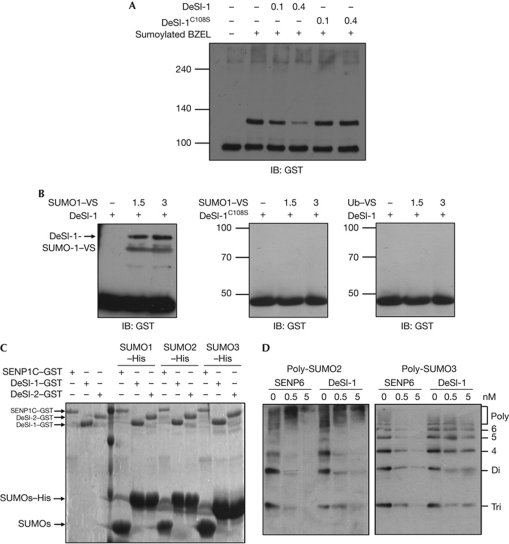
Figure 3.
In vitro desumoylation assay using recombinant DeSI-1 and BZEL from E. coli. (A) DeSI-1 desumoylates SUMO1-modified BZEL in vitro. A concentration of 10 μM of purified GST–BZEL fusion protein from E. coli was sumoylated in vitro. Subsequently, sumoylated BZEL was incubated with 0, 0.1 or 0.4 μM of DeSI-1or DeSI-1C108S mutant purified from E. coli for 1 h at 37°C. After incubation, the reaction mixture was analysed by immunoblotting with anti-GST antibody. The arrow indicates the position of sumoylated BZEL. (B) DeSI-1 formed covalent adducts with SUMO1-VS but not with Ub–VS. Recombinant purified GST–DeSI-1, or DeSI-1C108S mutant was incubated with 0, 1.5 and 3 μg each of SUMO1–VS or Ub–VS for 1 h at 37°C. After incubation, immunoblot analysis was performed with anti-GST antibody. (C) DeSI-1 lacks the SUMO-processing activity. A measure of 30 μg of SUMO precursors (SUMOs–His) was incubated for 1 h with 2 μg each of GST–DeSI-1, GST–DeSI-2 or GST–SENP1C purified from E. coli. Subsequently, the reaction mixtures were subjected to SDS–PAGE and stained with Coomassie blue. The size of the SUMO proteins generated by cleavage after the C-terminal diglycine motif is marked by an arrow. (D) DeSI-1 cleaves polymeric SUMO2/3 chains. A measure of 0.5 μg each of poly-SUMO2 or poly-SUMO3 chains was incubated with DeSI-1 or SENP6C at three different enzyme concentrations (0, 0.5 and 5 nM). Subsequently, the reaction mixtures were analysed by immunoblotting using anti-SUMO2/3 antibody. BZEL, BTB-ZF protein expressed in effector lymphocytes; DeSI-1, DeSumoylating Isopeptidase 1; E. coli, Escherichia coli; GST, glutathione S-transferase; His, histidine; IB, immunoblot; SENP1, sentrin-specific proteases 1; SUMO1, small ubiquitin-like modifier 1; Ub, ubiquitin; VS, vinyl sulphone.