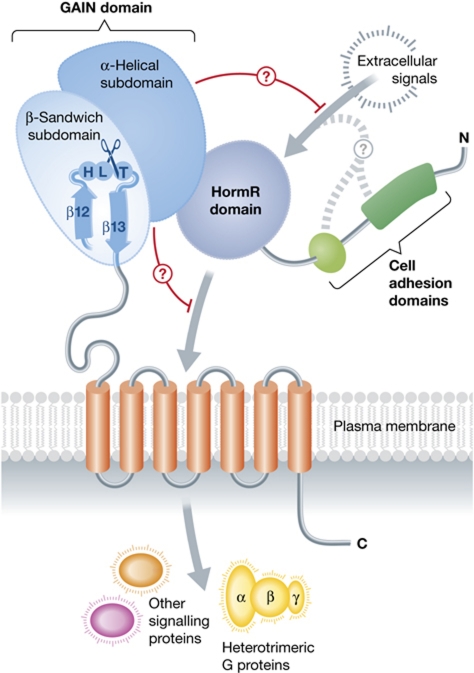
Figure 1.
The conserved structural core of the extracellular regions of cell-adhesion GPCRs, as revealed by new structures of latrophilin and BAI3. Arac et al revealed that regions formerly known as the stalk and the GPCR autoproteolyic site (GPS) fold into a single GAIN domain that is conserved in all 33 mammalian cell-adhesion GPCRs and in proteins related to PKD1. The GAIN domain catalyses its own proteolysis in a tight turn between the last two strands of the domain. The α-helical subdomain of the GAIN domains of latrophilin and BAI3 interacts with a HormR domain, although this domain is not found in all cell-adhesion GPCRs. However, this interdomain contact, and/or a direct interaction with the transmembrane helical bundle, may allow the GAIN domain to autoinhibit transmembrane signalling to heterotrimeric G proteins or other factors inside the cell.