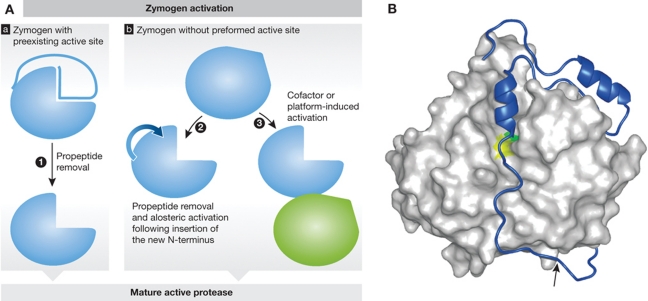
Figure 2.
Protease activation. (A) Schematic representation of protease zymogen activation with proteases where active site is preexisting (a) or non-existing (b). (1) Propeptide removal in proteases with preexisting active site such as seen in cysteine cathepsins (see B as an example); (2) allosteric or conformational activation of proteases with distorted active site, such as chymotrypsin-like serine proteases (granzymes and trypsin), and some caspases; (3) conformational rearrangement and formation of the active site upon cofactor or platform-based activation such as seen in caspases and during Factor VII activation. (B) Crystal structure of a zymogen form of a protease offers a major support in understanding the mechanism of zymogen activation. Procathepsin B is shown as an example of a protease with preexisting active site. Propeptide region of cysteine cathepsins covers the active site cleft of the enzyme thereby blocking access to substrates. The mature part of procathepsin B (Podobnik et al, 1997; 3PBH) is shown as a white surface, whereas the active site residues C29 and H199 areas are in yellow and green, respectively. The propeptide is shown as a blue ribbon with the autocatalytic cleavage site (M56-F57) marked with an arrow. Figure was prepared with MAIN (Turk, 1992) and rendered with POV-Ray.