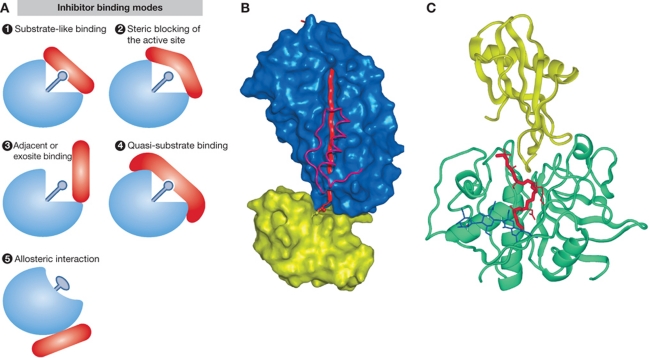
Figure 3.
Inhibitors, major regulators of protease activities. (A) Schematic representations of different modes of inhibitor binding (adapted from Bode and Huber, 2000). (1) Substrate-like binding with direct blockade of the protease active site, such as seen with most inhibitors of trypsin-like serine proteases (see B for trypsin–antitrypsin interaction as an example); (2) steric blocking of the active site such as seen with a number of cathepsin inhibitors (see C for stefin A–cathepsin H interaction as an example); (3) exosite binding such as seen with a number of thrombin inhibitors and in a more extreme case with XIAP binding to caspases-3 and -7, which is a combination of sterical blocking of the active site and exosite binding; (4) quasi-substrate-like binding such as seen with TIMP binding to MMPs; (5) allosteric inhibition through distortion of the active site such as seen with propeptides of chymotrypsin-like proteases. (B) Inhibition of serine proteases by a serpin as an example of substrate-like binding with direct blockade of the protease active site (A1). The crystal structure of antitrypsin is shown in complex with trypsin (Huntington et al, 2000; 1EZX) is shown as an example. In the first step of inhibition, the RSL of the serpin is cleaved and the P1 residue M358 remains covalently bound to the reactive site S195 of trypsin. In the next step, 15 residues of the reactive loop (orange strand) carrying trypsin are inserted into the middle of the β-sheet of the serpin molecule (blue surface), resulting in a movement of the trypsin molecule (yellow surface) from the top to the bottom of the serpin. Trypsin catalytic site is thereby distorted and partially unfolded. The inserted part of the RSL of serpin and helix F were removed from the serpin surface in order to make the insertion visible behind the scaffold of the helix F. The ester link atoms between the side chain trypsin S195 and carbonyl group serpin M358 are shown in ball-and-stick representation. (C) Inhibition of cysteine cathepsins by endogenous inhibitors. The crystal structure of stefin A in complex with cathepsin H (Jenko et al, 2003; 1NB3) is shown as an example of steric blocking of the active site (A2). The folds of stefin A and cathepsin H are shown in ribbon presentation. Stefin A is on the top and is shown in yellow, while cathepsin H is shown in cyan. The mini-chain of cathepsin H is shown as a red stick model, while the three visible carbohydrate rings are shown in blue.