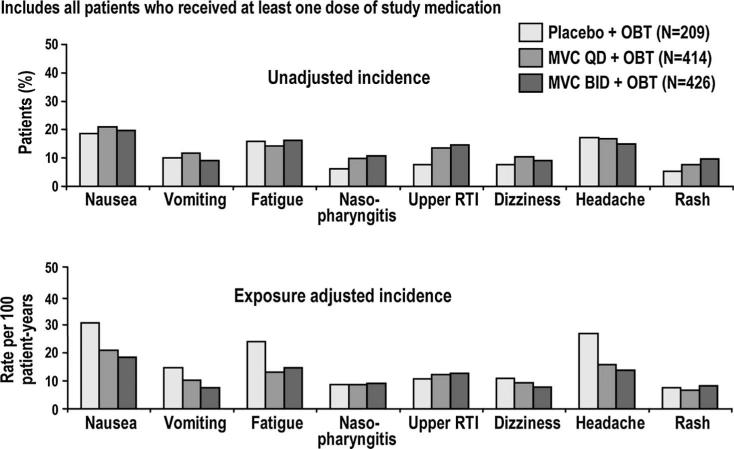
FIGURE 3.
Adverse events occurring in ≥5% of patients at end of blinded therapy. Includes all patients who received at least 1 dose of study medication. If the same patient in a given treatment had more than 1 occurrence in the same preferred term event category, only the most severe occurrence is taken. Event counts are adjusted to 100 years of patient exposure. Includes data up to 7 days after last dose of study drug. RTI, respiratory tract infection.