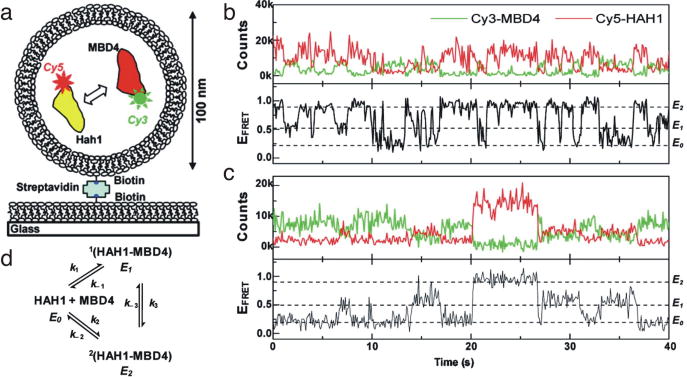
Fig. 9.
Single-molecule study of Hah1-MBD4 interactions. (a) Cy3-MBD4 and Cy5-Hah1 were encapsulated in 100 nm vesicles and immobilized to the slide. (b) Time traces from two individual vesicles that each contain one Cy3-MBD4 and one Cy5-Hah1. E0, ~0.2 FRETunbound state. E1, ~ 0.5 FRET bound state. E2, ~ 0.9 FRET bound state. (c) Hah1/MBD4 binding schematic. The determined rate constants are: k1 = 4.7 ± 0.7 x 105 M−1s−1, k−1 = 1.8 ± 0.1 s−1, k2 = 2.5 ± 0.4 x 105 M−1s−1, k−2 = 1.5 ± 0.1 s−1, k3 = 1.4 ± 0.2 s−1, k−.3 = 2.4 ± 0.3 s−1. Reprinted from (58) with permission from JACS.