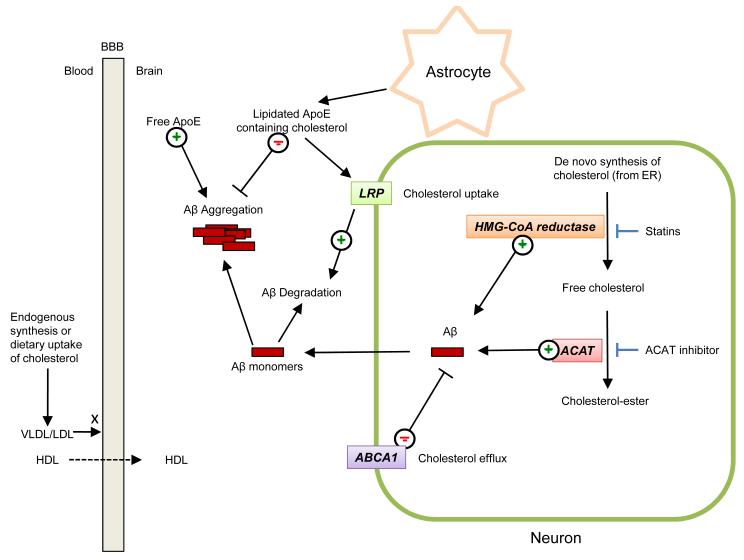
Figure 1. Contribution of cholesterol and apolipoprotein E metabolism to biogenesis, degradation and assembly of amyloid β-peptide.
Cholesterol in the brain is mainly derived from de novo synthesis from the endoplasmic reticulum. Small amounts of cholesterol can also be delivered to the brain from the periphery, via high density liproproteins (HDL), which can cross the blood-brain-barrier (BBB) unlike larger lipoproteins, such as low density lipoproteins (LDL) and very low density lipoproteins (VLDL). Hydroxymethyl glutaryl-coenzyme A (HMG-CoA) reductase mediates the rate-limiting step in de novo cholesterol biosynthesis. Excess free cholesterol is converted into cholesterol-ester by acyl-coenzyme A:cholesterol acyltransferase (ACAT). Inhibition of HMG-CoA reductase by statins leads to decreased levels of Aβ in animal models and ACAT inhibition has been also shown to reduce Aβ levels by a mechanism that remains as yet poorly defined. ApoE-containing HDL-like particles inhibit the aggregation of Aβ, while free ApoE has been shown to promote Aβ aggregation. LDL receptor-related protein (LRP) serves as a neuronal receptor for astrocyte-produced ApoE-containing lipid particles, thus mediating their internalization into neurons where they are broken down. ApoE-containing HDL-like particles inhibit the aggregation of Aβ, while free ApoE has been shown to promote Aβ aggregation in vitro. ABAC1, a regulator of cholesterol efflux, has been also shown to modulate Aβ levels in neurons. For simplicity, Aβ is drawn as free-floating in the cytoplasm, although it is produced in the lumen of neuronal organelles of the late secretory and endolysosomal systems, from where it can be secreted.