Abstract
E2-EPF ubiquitin carrier protein (UCP) stabilizes hypoxia-inducible factor-1α (HIF-1α) inducing ischemic vascular responses. Here, we investigated the effect of UCP gene transfer on therapeutic angiogenesis. Adenovirus-encoded UCP (Ad-F-UCP) increased the expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) in cells and mice. Conditioned media from UCP-overexpressing cells promoted proliferation, tubule formation, and invasion of human umbilical-vascular-endothelial cells (HUVECs), and vascularization in chorioallantoic membrane (CAM) assay. Ad-F-UCP increased the vessel density in the Martigel plug assay, and generated copious vessel-like structures in the explanted muscle. The UCP effect on angiogenesis was dependent on VEGF and FGF-2. In mouse hindlimb ischemia model (N = 30/group), autoamputation (limb loss) occurred in 87% and 68% of the mice with saline and Ad encoding β-galactosidase (Ad-LacZ), respectively, whereas only 23% of the mice injected with Ad-F-UCP showed autoamputation after 21 days of treatment. Ad-F-UCP increased protein levels of HIF-1α, platelet-endothelial cell adhesion molecule-1 (PECAM-1), smooth muscle cell actin (SMA) in the ischemic muscle, and augmented blood vessels doubly positive for PECAM-1 and SMA. Consequently, UCP gene transfer prevented muscle degeneration and autoamputation of ischemic limb. The results suggest that E2-EPF UCP may be a target for therapeutic angiogenesis.
Introduction
Angiogenic factors have been extensively exploited for the gene therapy of coronary and peripheral artery disease including critical limb ischemia (CLI).1 Therapeutic effect of angiogenic factors, such as vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs), has been verified in preclinical animal models, but appears to be marginal in clinical trials.1 Thus, novel modality for therapeutic angiogenesis needs to be sought.
Blood vessel networks mature through a series of multiple steps including numerous factors.2 Hypoxia-inducible factor-1α (HIF-1α) associates with HIF-1β to form HIF-1 transcription factor that activates the expression of various genes involved in angiogenesis.3,4 For these reasons, HIF-1α has been extensively examined for its potential for therapeutic angiogenesis.5,6 E2-EPF ubiquitin carrier protein (UCP) promotes the ubiquitin-mediated proteolysis of von Hippel–Lindau (VHL) that is part of E3 ubiquitin ligase complex.7,8 The VHL E3 ubiquitin ligase targets HIF-1α for ubiquitination and degradation in an oxygen-dependent manner.9,10 Therefore, HIF-1α is stabilized under hypoxia and has been shown to be a master regulator for hypoxic/ischemic vascular responses.6 Angiogenesis and particularly arteriogenesis should also be induced in normoxic area around the ischemic tissue for therapeutic neovascularization.11 Forced expression of UCP destabilizes VHL and stabilizes HIF-1α in cells under normoxia and under hypoxic (1% oxygen) condition.7 This ability of UCP to stabilize HIF-1α led us to test whether UCP is a target for therapeutic angiogenesis.
Results
UCP gene transfer promotes the expression and secretion of VEGF and FGF-2 in cells
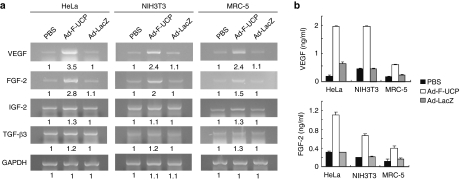
We showed previously that adenovirus-encoded UCP (Ad-F-UCP) increases the expression of VEGF through the VHL/HIF pathway at mRNA level.7 Hypoxic induction of an HIF-1α-dependent FGF-2 autocrine loop promotes angiogenesis in human endothelial cells.12 Insulin-like growth factor-2 and transforming growth factor-β3 genes are activated by HIF-1 and involved in blood vessel formation.13,14 Gene expression of angiogenic factors by HIF-1 is regulated in a cell type–specific manner.4 Based on these previous findings, we examined whether Ad-F-UCP increased the expression of those angiogenic factors in the cell lines of different origin (Figure 1a). VEGF and FGF-2 transcripts were substantially increased in all the cell lines transduced with Ad-F-UCP compared to those with phosphate-buffered saline (PBS) or Ad encoding β-galactosidase (Ad-LacZ). Because insulin-like growth factor-2 and transforming growth factor-β3 transcripts appeared not to be greatly increased in the cells with Ad-F-UCP (Figure 1a), we quantified protein levels of only VEGF and FGF-2 in the culture supernatants (Figure 1b). UCP expression enhanced the secretion of VEGF and FGF-2 in the three cell lines.
Figure 1.
Adenovirus-encoded UCP (Ad-F-UCP) promotes expression and secretion of VEGF and FGF-2 in cells. (a) Cells were transduced with phosphate-buffered saline (PBS) with or without Ad-F-UCP or Ad encoding β-galactosidase (Ad-LacZ) at multiplicity of infection of 100 (HeLa and MRC-5 cells) or 200 (NIH3T3 cells), and incubated in a serum-free medium. Culture supernatants were saved for enzyme-linked immunosorbent assay (ELISA) and total RNAs were prepared from the cells at 48 hours after transduction. Reverse transcription-polymerase chain reaction (RT-PCR) was performed with total RNAs and relevant primers. The RT-PCR products were separated by a 1% agarose gel electrophoresis, visualized by ethidium bromide, and quantified by densitometry. The band intensity is relatively expressed. (b) ELISA was performed with the culture supernatants three times, each in duplicate. Data are mean ± SD. FGF-2, fibroblast growth factor-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGF-2, insulin-like growth factor-2; TGF-β3, transforming growth factor-β3; VEGF, vascular endothelial growth factor.
Conditioned media from UCP-overexpressing cells promote proliferation, tubule formation, and invasiveness of HUVECs
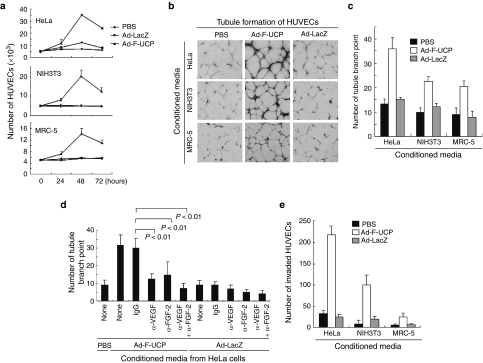
We collected conditioned media from HeLa, NIH3T3, and MRC-5 cells with or without UCP gene transfer and incubated human umbilical-vascular-endothelial cells (HUVECs) in the conditioned media for 3 days and counted them once a day. We photographed viable HUVECs at 48 hours after incubation and stained them with crystal violet (Supplementary Figure S1a,b). The conditioned media from cells with Ad-F-UCP, but not PBS or Ad-LacZ enhanced the proliferation of HUVECs (Figure 2a). This proliferation-enhancing effect was in the order of the conditioned medium from HeLa, NIH3T3, and MRC-5 cells with Ad-F-UCP (Figure 2a), which appeared to correlate with protein levels of VEGF and FGF-2 in the culture supernatants (Figure 1b).
Figure 2.
Ad-F-UCP enhances proliferation, tubule formation, and invasion of HUVECs. (a) Cells were transduced with or without Ad-F-UCP or Ad-LacZ at a multiplicity of infection (MOI) of 100 for HeLa and MRC-5 cells or an MOI of 200 for NIH3T3 cells, incubated in a serum-free medium, and culture supernatants were collected (conditioned media). HUVECs were incubated in the conditioned media for the indicated times and counted with a hemocytometer. This assay was performed three times, each in triplicate. (b,c) HUVECs were incubated for 24 hours in the conditioned media as described in (a), stained with 0.05% crystal violet, and photographed with a microscope at ×100 magnification (b). (c) Tubule branch point was counted in five independent fields per group in three separate experiments. (d) The conditioned media was prepared from HeLa cells with or without Ad-F-UCP or Ad-LacZ at an MOI of 100. They were preincubated with or without 5 µg/ml anti-VEGF and/or 2 µg/ml anti-FGF-2 antibodies, or IgG for 24 hours at 4°C, and HUVECs were incubated in the preincubated media for 24 hours. Total amount of antibody was adjusted to 7 µg/ml by the addition of IgG. HUVECs were stained with 0.05% crystal violet and then photographed (Supplementary Figure S2). The branch point was counted in five representative fields per group in three separate experiments. (e) Invasion assay was performed with the conditioned media from cells as described in (a). Cells were stained with hematoxylin and eosin and photographed (Supplementary Figure S3). The permeating HUVECs were counted in five representative fields per group in three separate experiments. Data are mean ± SD (a,c,d,e). Ad-LacZ, adenovirus encoding β-galactosidase; Ad-F-UCP, Ad-encoded UCP; FGF, fibroblast growth factor; HUVEC, human umbilical-vascular-endothelial cell; PBS, phosphate-buffered saline; VEGF, vascular endothelial growth factor.
Tubule formation assay revealed that the conditioned media from cells with Ad-F-UCP promoted the formation of elongated and robust tube-like structures, and increased tubule branch point more than twofold compared with the controls (Figure 2b,c). To examine the involvement of VEGF and FGF-2 in the tubule formation, we incubated HUVECs in the conditioned media with or without neutralizing antibodies against VEGF or FGF-2 (Figure 2d). The branch point increased by the conditioned media from the cells with Ad-F-UCP was significantly reduced by the VEGF or FGF-2 antibody (Figure 2d), and further decreased when both the antibodies were together present.
The invasion assay revealed that the conditioned media from cells transduced with Ad-F-UCP markedly increased the invasiveness of HUVECs compared with the controls (Figure 2e).
UCP gene transfer stimulates angiogenesis in vivo
We examined the in vivo angiogenic potential of UCP gene transfer by three methods: chorioallantoic membrane (CAM),15 Matrigel plug,16 and ex vivo skeletal muscle angiogenesis assays.17
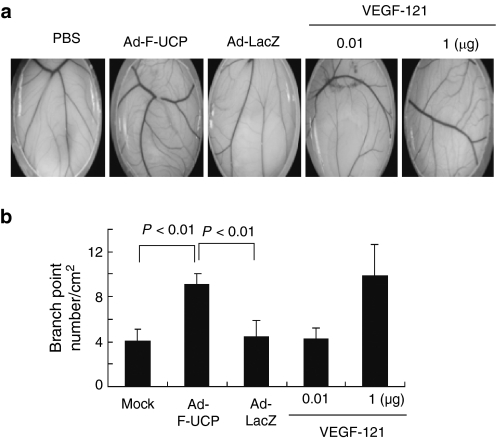
Chicken CAM assay showed that the conditioned medium from HeLa cells transduced with Ad-F-UCP significantly increased the number of branch points compared with that from cells with PBS or Ad-LacZ (Figure 3a,b). The conditioned medium from cells with Ad-F-UCP exhibited angiogenic effect comparable to 1 µg of human recombinant VEGF-121. This potent effect of the conditioned medium may result from the fact that it contains VEGF, FGF-2, and other angiogenic factors, which may synergistically promote angiogenesis.
Figure 3.
Ad-F-UCP induces neovascularization in chorioallantoic membrane (CAM) assay. (a) The conditioned media was prepared from HeLa cells with or without Ad-F-UCP or Ad-LacZ at a multiplicity of infection of 100 and enriched up to 50-fold. Cover glasses were coated with the enriched media or indicated amounts of recombinant human VEGF-121 and put on the CAM of fertilized eggs. Seven days later, images were taken with a microscope at ×10 magnification. (b) The branch point from five images per experimental group was counted. Data are mean ± SD. Ad-LacZ, adenovirus encoding β-galactosidase; Ad-F-UCP, Ad-encoded UCP; PBS, phosphate-buffered saline; VEGF, vascular endothelial growth factor
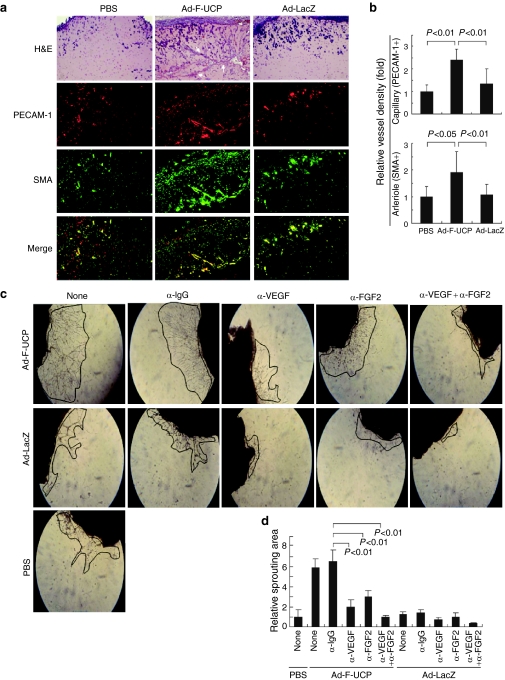
We performed the Matrigel plug assay with NIH3T3 cells with or without Ad-F-UCP. Hematoxylin and eosin (H&E) staining of the Matrigel plug sections showed that the blood vessel was more abundantly generated in the Ad-F-UCP group (Figure 4a). Physiological maturation of blood vessel requires recruitment of smooth muscle cells representing pericytes. Thus, lining of smooth muscle cells with platelet-endothelial cell adhesion molecule-1 (PECAM-1)–positive cells represents maturation of blood vessel.2,18 Immunofluorescent staining of the plug sections revealed that the PECAM-1-positive area representing the endothelium was lined with smooth muscle cell actin (SMA)–positive area (Figure 4a). This lining was more abundantly detected in the plug section with Ad-F-UCP compared to that with PBS or Ad-LacZ (Figure 4a Merge, yellow). Ad-F-UCP increased the capillary (PECAM-1 positivity) and mature vessel (SMA-positivity) densities about twofold in the plug assay, respectively, compared with the controls (Figure 4b).
Figure 4.
Ad-F-UCP induces neovascularization in vivo. (a) NIH3T3 cells were transduced with or without Ad-F-UCP or Ad-LacZ at a multiplicity of infection of 200, mixed the cells with Matrigel, and injected 0.5 ml of each mixture subcutaneously into the right abdominal surface of mice (N = 3/group). Matrigel plugs were removed at 5 days after injection. Plug sections were stained with H&E or indicated antibodies and photographed under a microscope (×100). Representative photographs are shown. Arrows indicate blood vessel. (b) ImageJ software was used to quantify the vessel density based on PECAM-1-positive and SMA-positive areas in (a). Vessel density of PBS group is arbitrarily defined as 1 and expressed relatively. Data are mean ± SD from five randomly chosen fields per group. (c,d) Skeletal thigh muscles of mice (N = 3/group) were injected with or without Ad-F-UCP or Ad-LacZ. Excised muscles were covered with Dulbecco's modified Eagle's medium containing 5% fetal bovine serum with or without 10 µg/ml VEGF and/or 5 µg/ml FGF-2 antibodies or IgG. Total amount of antibody was adjusted to 15 µg/ml by the addition of IgG. The medium was changed with fresh one containing each antibody at the same concentration every other day. Outgrowth of vessel-like structures was photographed on day 7 with a microscope (×50) and was marked by the line. (c) Representative photographs are shown. (d) A picture of the whole explant was taken, area of visible vessel-like structures was marked, and planar sprouting area was quantified by densitometry. Data are mean ± SD from five images per group in three separate experiments. Sprouting area of the muscle with PBS is arbitrarily defined as 1. Ad-LacZ, adenovirus encoding β-galactosidase; Ad-F-UCP, Ad-encoded UCP; FGF, fibroblast growth factor; H&E, hematoxylin and eosin; PBS, phosphate-buffered saline; PECAM-1, platelet-endothelial cell adhesion molecule-1; SMA, smooth muscle cell actin; VEGF, vascular endothelial growth factor.
We confirmed that the intramuscular injection of Ad-F-UCP decreased VHL level, increased HIF-1α level and mRNA levels of VEGF and FGF-2 in the thigh muscle of mouse (Supplementary Figure S4a,b). We then performed ex vivo skeletal muscle angiogenesis assay.17 The formation of sprouted vessel-like structures were more in the Ad-F-UCP group compared with the Ad-LacZ group (Figure 4c), and were increased in an incubation time–dependent manner (Supplementary Figure S4c,d) and in an Ad-F-UCP-dosage-dependent manner (Supplementary Figure S4e,f). To test the involvement of VEGF or FGF-2 in the formation of vessel-like structures, skeletal muscles were cultured in the presence or absence of anti-VEGF and/or anti-FGF-2 antibodies. Anti-VEGF or anti-FGF-2 antibody significantly inhibited Ad-F-UCP-mediated outgrowth of vessel-like structures (Figure 4c,d), and the sprouting was more strongly inhibited when both antibodies were present. Collectively, the results suggest that UCP gene transfer promotes neovascularization in vivo.
UCP gene transfer prevents loss of ischemic mouse hindlimb
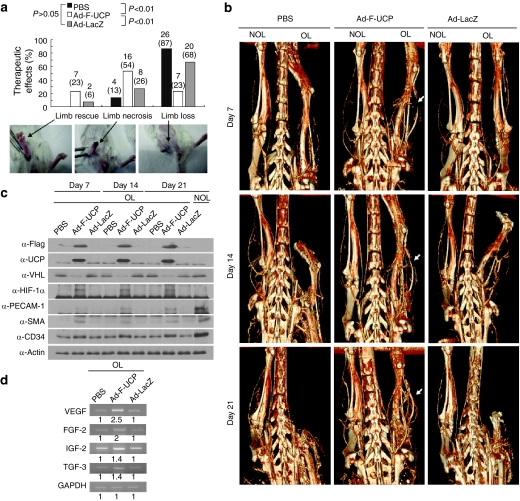
To examine whether our surgical procedure indeed led to ischemic hindlimb, we visualized hindlimb vasculature by micro-computed tomography (CT) after femoral artery occlusion19 and also examined the protein level of HIF-1α. Blood perfusion in the operated leg was drastically reduced compared with that in the non-operated leg or in the leg before operation (Supplementary Figure S5a). At 12 hours after the surgery, HIF-1α protein was detected in the thigh muscle of the operated leg, but not the non-operated leg (Supplementary Figure S5b), suggesting the successful induction of ischemic tissue due to insufficient blood perfusion by the surgery. We then evaluated the therapeutic effects of Ad-F-UCP (Figure 5a). Limb loss occurred in 87% and 68% of the mice with PBS and Ad-LacZ, respectively, whereas only 23% of the mice injected with Ad-F-UCP showed limb loss. Limb rescue and necrosis were observed in 23% and 54% of the mice with Ad-F-UCP, respectively, while 6% and 26% of the mice with Ad-LacZ exhibited limb rescue and limb necrosis, respectively. In the PBS group, no mice recovered and 13% of the mice had limb necrosis. These differences in the therapeutic effect between the Ad-F-UCP group and the control groups were significant. The results suggest that UCP gene transfer delays limb loss.
Figure 5.
Ad-F-UCP prevents autoamputation in the ischemic limb of mouse. (a) At day 21 after injection with or without Ad-F-UCP or Ad-LacZ (N = 30 mice/group) into the ischemic muscle, treatment outcome was divided into three grades based on the physiological status of the ischemic hindlimb: limb rescue, limb necrosis, and limb loss. Limb rescue means no physical and functional defect of the operated leg (OL). Limb necrosis indicates the case that foot or toe is impaired but knee is intact, while limb loss (autoamputation) means loss of limb above knee. Arrows indicate the representative images for each status. Therapeutic effects were summed up from six separate experiments. (b) Micro-computed tomography (CT) images of operated leg (OL) and non-operated leg (NOL) were taken at the indicated times after treatment as described in the Materials and Methods section. Representative micro-CT images with one mouse per group in six separate experiments are shown. Arrow indicates increased vascularity in the Ad-F-UCP group. (c) The ischemic limb was treated as described in the Materials and Methods section and the indicated proteins in the thigh muscle were analyzed at the indicated times by immunoblotting. These experiments were performed with one mouse per group in two independent animal experiments and the representative is shown. (d) The ischemic limb was treated as described in Materials and Methods section and reverse transcription-polymerase chain reaction (RT-PCR) was performed with total RNAs from the thigh muscle after 21 days of treatment. RT-PCR products were resolved by a 1% agarose gel electrophoresis and visualized by ethidium bromide. Band intensity was quantified by densitometry and is relatively expressed. Similar results were obtained from five separate animal experiments and the representative is shown. The samples in (c) and (d) were taken from the thigh muscles of mice with limb necrosis in PBS and Ad-LacZ groups and from those with limb necrosis or rescue in Ad-F-UCP group. Ad-LacZ, adenovirus encoding β-galactosidase; Ad-F-UCP, Ad-encoded UCP; FGF, fibroblast growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; H&E, hematoxylin and eosin; HIF, hypoxia-inducible factor; IGF-2, insulin-like growth factor-2; PBS, phosphate-buffered saline; PECAM-1, platelet-endothelial cell adhesion molecule-1; SMA, smooth muscle cell actin; TGF-β3, transforming growth factor-β3; VEGF, vascular endothelial growth factor; VHL, von Hippel–Lindau.
To examine whether Ad-F-UCP-mediated therapeutic effects correlated with blood perfusion in ischemic tissue, hindlimb vasculature was imaged using micro-CT once a week after treatment (Figure 5b). Vascularity in ischemic hindlimb was severely impaired in mice injected with PBS or Ad-LacZ at day 7 after treatment and eventually limb loss occurred at day 21, while Ad-F-UCP gradually increased vascularity at the hindlimb distal to the gene transfer site and rescued ischemic hindlimb. Blood vessel network was reconstituted in the ischemic hindlimb with Ad-F-UCP after 21 days of treatment, which was comparable to that in the non-operated leg (Figure 5b).
F-UCP was expressed for 21 days in the ischemic thigh muscle with Ad-F-UCP, resulting in a decrease in VHL level and an increase in HIF-1α protein level (Figure 5c). Expression of VEGF and FGF-2 was increased more than twofold in the thigh muscle with Ad-F-UCP at day 21 after injection, compared to that with PBS or Ad-LacZ (Figure 5d). Protein levels of PECAM-1 and SMA were highly maintained for 21 days in the thigh muscle with Ad-F-UCP, although their levels were lower than those in the thigh muscle without operation (Figure 5c). Protein level of a progenitor cell marker CD34 was also slightly increased in the thigh muscle with Ad-F-UCP for 21 days (Figure 5c), suggesting that the endothelial progenitor cells may be involved in the UCP-mediated neovascularization.
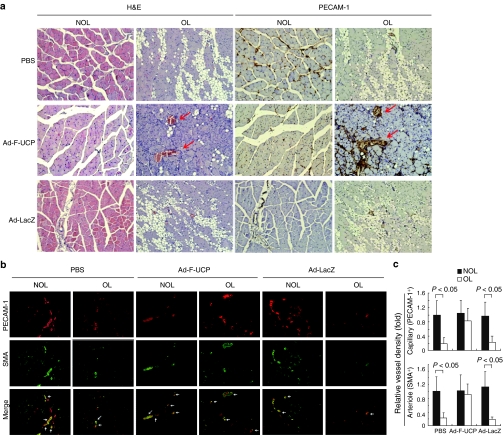
Immunohistochemical analysis revealed that the ischemic muscle was massively degenerated in the PBS or Ad-LacZ group, but not with Ad-F-UCP (Figure 6a, H&E). PECAM-1-positive area (Figure 6a, dark brown) was more abundantly detected in the ischemic muscle with Ad-F-UCP, and was frequently overlapped with SMA-positive one in the ischemic muscle with the Ad-F-UCP group (Figure 6b Merge, yellow), suggesting efficient maturation of blood vessels by the UCP gene transfer. Capillary (PECAM-1 positivity) and mature vessel (SMA-positivity) densities were significantly decreased in the ischemic limb with PBS or Ad-LacZ, but not with Ad-F-UCP compared with those in nonischemic limb (Figure 6c). Collectively, therapeutic outcome of the UCP gene transfer (Figure 5a) is supported by the facts that Ad-F-UCP gene transfer induced angiogenic factors (Figure 5d), maintained vessel markers at high level (Figure 5c), increased vessel density and maturation (Figure 6b,c), and promoted muscle regeneration (Figure 6a) in the ischemic muscle.
Figure 6.
Ad-F-UCP promotes muscle regeneration and vessel maturation. (a,b) The ischemic limb of the mouse was treated as described in Materials and Methods section, and the thigh muscles of the mice with limb necrosis in PBS and Ad-LacZ groups and those of mice with limb necrosis or rescue in Ad-F-UCP group was excised after 21 days of treatment. H&E, diaminobenzidine (a, ×200), or immunofluorescent (b, ×100) staining was performed with muscle sections and photographed under a microscope. Representative photographs are shown. Arrows in (a) and (b, Merge) indicate blood vessel and more mature vessels doubly positive for PECAM-1 and SMA, respectively. (c) ImageJ software was used to quantify the vessel density based on PECAM-1-positive and SMA-positive areas in (b). Vessel density of the PBS group is arbitrarily defined as 1. Data are mean ± SD from five independent fields per group in three separate experiments. Ad-LacZ, adenovirus encoding β-galactosidase; Ad-F-UCP, Ad-encoded UCP; H&E, hematoxylin and eosin; NOL, non-operated leg; OL, operated leg; PBS, phosphate-buffered saline; PECAM-1, platelet-endothelial cell adhesion molecule-1; SMA, smooth muscle cell actin.
Discussion
Here, we demonstrated that E2-EPF UCP gene transfer increases the expression of VEGF and FGF-2 through the VHL/HIF pathway in cells and mice, and thereby significantly prevents autoamputation in the mouse hindlimb ischemia.
VEGF, PDGF-B, or FGF-2 plays important roles in therapeutic angiogenesis.20,21 Although FGF-2 or PDGF-B is activated under hypoxic condition,4,22 it is unknown whether HIF-1 directly regulates their expression. We found that UCP induces the gene expression of VEGF and FGF-2 (Figures 1 and 5c,d; Supplementary Figure S4a,b). However, we could not detect increased expression of PDGF-B in the UCP-overexpressing cells. This may result from the fact that the HIF-1-mediated transcriptional response to hypoxia is cell type specific, as previously reported.4 Our result is consistent with the previous finding that silencing of prolyl hydroxylase domain-2 gene stabilizes HIF-1α and thereby induces the expression of VEGF and FGF-2, but not PDGF-B.22 VEGF expression is directly regulated by HIF-1 through the hypoxia-response element in its promoter.23 Thus, although it remains to be determined whether HIF-1α directly activates FGF-2 expression, our finding suggests that HIF-1α positively regulates FGF-2 expression under nonhypoxic conditions.
Endogenous HIF-1α was detected in the ischemic muscle at 12 hours after femoral artery occlusion most likely because of insufficient blood flow (Supplementary Figure S5b). HIF-1α was not detected in the ischemic muscle with PBS or Ad-LacZ at day 8 after surgery (Figure 5c). Ad-F-UCP increased HIF-1α in the ischemic muscle and this effect of UCP on HIF-1α level was maintained for 21 days after injection (Figure 5c). These results suggest that the ischemic muscle was no more hypoxic at day 8 after the surgery and that UCP gene transfer but not ischemia due to the surgery stabilizes HIF-1α in the ischemic muscle, resulting in increased expression of VEGF and FGF-2 (Figure 5d). Increased HIF-1α level was detected in the thigh muscle of the mouse at day 3 after intramuscular injection of Ad-F-UCP, resulting in the increased expression of VEGF and FGF-2 (Supplementary Figure S4a,b). Neutralizing anti-VEGF and anti-FGF-2 antibodies abolished the ability of UCP to promote the tubule formation of HUVECs and outgrowth of vessel-like structures in the explanted muscle (Figures 2d and 4c,d). These results suggest that UCP promotes in vivo vascular responses by stabilizing HIF-1α and exhibits its effect on angiogenesis mainly through VEGF and FGF-2, although we cannot exclude the involvement of other angiogenic factors.
Ad-F-UCP significantly prevented autoamputation and rescued ischemic limb more effectively than Ad-LacZ (Figure 5a). Nevertheless, limb rescue was detected in 2 of the 30 mice injected with Ad-LacZ but not in mice with PBS, and limb loss occurred in the mice with PBS (87%) more frequently than in those with Ad-LacZ (68%), although the difference between the two control groups was insignificant (Figure 5a). These results may suggest a positive effect of adenoviral vector itself on therapeutic angiogenesis. This vector effect was previously observed in animal model.24 Adenoviral vector was shown to induce inflammatory reaction in the host.25 Inflammation was shown to contribute to neovascularization.26 Thus, it is possible that adenoviral vector-mediated stimulation of inflammatory response may in part help to promote therapeutic angiogenesis by UCP.
Protein level of endogenous VHL was decreased for 21 days in the ischemic muscle with Ad-F-UCP (Figure 5c). The ischemic muscle cells with Ad-F-UCP were regenerated as much as those of nonischemic ones, while the muscle cells were massively degenerated in the PBS or Ad-LacZ group (Figure 6a, H&E). Thus, it is possible that a decrease in VHL level may help to regenerate ischemic muscle and promote neovascularization. In this aspect, therapeutic effect of UCP gene transfer may be substantially different from that of gene transfer of constitutively active HIF-1α mutants, which does not greatly affect VHL level. However, because VHL is tumor suppressive,27 long-term effect of UCP gene transfer remains to be investigated.
At day 8 after femoral artery excision, autoamputation already occurred in 41% and 32% of the ischemic limb with PBS and Ad-LacZ, respectively, while 10% of the ischemic limb with Ad-F-UCP exhibited limb loss. Thus, it would be improper to assess therapeutic effect of UCP gene transfer by measuring lower extremity blood flow if autoamputaion frequently occurs at early times after induction of limb ischemia. Furthermore, laser Doppler imaging, which has been commonly used for the measurement of blood flow, assesses only surface/skin blood flow, but not an accurate tissue perfusion. For these reasons, we evaluated the therapeutic effects of UCP gene transfer on the three phenotypes of ischemic limb rather than the measurement of blood flow (Figure 5a). In particular, we focused on how effectively UCP gene transfer could prevent limb loss in acute ischemic model. This acute ischemic model may not properly reflect clinical situation of CLI patients.28 Nevertheless, acute ischemic model would be appropriate to clearly and cost-effectively assess the therapeutic outcome of angiogenic gene transfer in a relatively short period of time. One of the current aims of management of patients with CLI is to prevent amputation.28 In CLI patients, FGF-1 gene transfer results in a significantly reduced risk of major amputation.29 This finding supports that our assessment on therapeutic effects of UCP gene transfer may be more realistic than the measurement of blood flow recovery in ischemic limb.
In summary, the results of this study provide proof of principle that UCP gene transfer induces expression of angiogenic factors through stabilization of HIF-1α in cells and mice, thereby reconstitutes blood vessel network effectively and prevents autoamputation in the mouse hindlimb ischemia. Our results suggest that UCP gene transfer may help to facilitate amputation-free survival of CLI patients.
Materials and Methods
Cell culture. HUVECs were maintained in a standard endothelial cell culture medium (EGM-2 BulletKit; Clonetics, San Diego, CA). HUVECs with passage 4–8 were used for the experiments. The endothelial cell culture medium was switched to a serum-free Dulbecco's modified Eagle's medium at 24 hours before assay. HeLa carcinoma and human MRC-5 and mouse NIH3T3 fibroblast cells were cultured under a standard condition.
Ad vector and transduction. Construction, amplification, titer determination, and transduction of Ad-F-UCP or Ad-LacZ were done as previously described.7
Recombinant proteins and antibodies. We purchased antibodies specific for Flag, actin, SMA (Sigma-Aldrich, St Louis, MO), CD34 (Santa Cruz Biotechnology, Santa Cruz, CA), VHL, HIF-1α, PECAM-1 (BD Pharmingen, San Diego, CA), and human VEGF and FGF-2 (Santa Cruz Biotechnology), complete proteinase inhibitor cocktail (Roche, Basel, Switzerland), VEGF-121 (R&D Systems, Minneapolis, MN), and IgG (Jackson Laboratories, Bar Harbor, ME). We generated UCP-specific antibody by immunizing mice with His-UCP.
Enzyme-linked immunosorbent assay. Protein levels in culture supernatants were quantified by enzyme-linked immunosorbent assay using human or mouse VEGF and FGF-2 Quantikine kits according to the manufacturer's instructions (R&D Systems).
RNA extraction and reverse transcription-polymerase chain reaction. Total RNA was extracted from cells or muscles using easy-spin RNA extraction kit (Intron, Seoul, Korea). Complementary DNA synthesis and polymerase chain reaction were performed using one-step master mix (Intron). Complementary DNA was synthesized from 1 µg of total RNA and amplified with polymerase chain reaction using primers specific for each gene. Glyceraldehyde 3-phosphate dehydrogenase transcript was used to normalize sample amplification. Band intensity was quantified using densitometer software. Primer sequences for reverse transcription-polymerase chain reaction are shown in Supplementary Figure S6.
Protein extraction and immunoblotting. We lysed cells in a buffer (50 mmol/l Tris-HCl, pH 7.5, 150 mmol/l NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, one tablet proteinase inhibitor cocktail/100 ml). We homogenized tissues in PRO-PREP lysis buffer (Intron) and prepared protein samples by clearing cell lysates or homogenates by centrifugation. Immunoblotting was performed as previously reported.7,8 Protein bands were visualized by the ECL detection system (Intron). α-Actin-specific antibody was used for a loading control.
HUVEC proliferation, tubule formation, and invasion assays. Conditioned media were prepared from cells with or without adenoviral vectors at 48 hours after transduction. HUVECs were plated at 5 × 10³ cells/well in the conditioned media on 24-well plates. Viable cells were counted with a hemocytometer.
Matrigel (BD Bioscience, Bedford, MA) was added to a 12-well plate, which was then incubated for 30 minutes at room temperature for the matrix solution to be solidified. HUVECs (2 × 105) per well were then plated on top of the solidified matrix, and cultured at 37°C for 24 hours. HUVECs were fixed with methanol for 15 minutes and stained with 0.05% crystal violet. Tubule formation was inspected with a microscope. The number of tubule branch point in microscopic field (×100) was determined.
HUVECs were plated at a density of 1 × 104 cells/well in a serum-free Dulbecco's modified Eagle's medium in the upper chamber in Transwell chambers (8 µm, 24-well format). The insert membranes were coated with Matrigel. Conditioned medium was added to the lower chamber. After HUVECs were cultured for 24 hours, the insert membranes were cut. HUVECs were stained with H&E.
CAM assay. Fresh fertilized eggs were incubated in a standard egg incubator at 37°C. A small hole (~1.5 cm in diameter) was made by removing the egg shell and inner shell membrane. The exposed area was then sealed with cellophane tape at day 3 after incubation. The eggs were incubated with the hole upright. Sterile cover glasses were absorbed with 10 µl of the enriched media, and placed on the CAMs that had been preincubated for 3 days. The CAM was photographed under a microscope. The number of vessel branch point was counted within identical field.
Immunohistochemistry. Matrigel plugs or muscle tissues were frozen in optimal cutting temperature compound or fixed with 10% formalin for H&E staining or immunohistochemistry. Frozen samples were sectioned at a thickness of 5–7 µm in a cryostat. Sections were fixed with methanol, and reacted with a mixture of anti-PECAM-1 and fluorescein isothiocyanate–conjugated anti-SMA monoclonal antibodies. Sections were subsequently reacted with rhodamine-conjugated anti-mouse IgG antibody (Sigma-Aldrich).
For diaminobenzidine staining, paraffin-embedded tissue sections were deparaffinized and hydrated through xylenes and graded alcohol series. Antigen retrieval was performed at 55°C for 10 minutes using protease K (10 µg/slide). Endogenous peroxidase activity was quenched by treating the sections with 0.3% H2O2 for 30 minutes. The sections were incubated in PBS with Tween-20 (0.05% Tween-20 in PBS) containing 3% fetal bovine serum at room temperature for 30 minutes. Envision Dual link system-HRP kit (Dako, Carpinteria, CA) was used for diaminobenzidine staining.
Ex vivo angiogenesis assay. We injected 2 × 108 plaque-forming-unit (PFU) of Ad-LacZ or Ad-F-UCP in 100 µl of PBS into skeletal muscle of mouse. Three days after injection, the muscles were excised and cut in the middle to expose the injection area and washed three times with PBS. The washed muscles were placed in a 24-well plate containing 250 µl of Matrigel and incubated at 37°C for 30 minutes to solidify the gel. Samples were then covered with 500 µl of Dulbecco's modified Eagle's medium containing 5% fetal bovine serum. The plate was placed in a 5% CO2 atmosphere at 37°C. Outgrowth of vessel-like structures was observed with a microscope (×50).
Mouse hindlimb ischemia model. Mouse experiments were performed in accordance with the guidelines and under the approval of the Institutional Review Committee for the Animal Care and Use, KRIBB, Daejeon, Korea. We used 8- to 10-week-old BAL b/c female mice weighing 20–25 g. Mice underwent surgical ligation of the part of the right proximal femoral artery and distal artery site and excision, as previously reported.30 One day after the surgery, 2 × 109 PFU of Ad-F-UCP or Ad-LacZ in 100 µl of PBS were injected into four different sites on skeletal muscle of mouse. The left leg was left without operation and was used as an internal control. Experiment with 5–13 mice/group was repeated six times. One identical mouse in each group per experiment was used for hindlimb vasculature imaging at 7, 14, and 21 days after treatment. One or two mice in each group per experiment were killed for immunoblotting, reverse transcription-polymerase chain reaction, and immunohistochemical analyses at 7 and 14 days after treatment, respectively. After 21 days of treatment, remaining mice were analyzed for therapeutic effects, killed, and then used for biochemical and immunological analyses.
Micro-CT imaging of mouse hindlimb vasculature. Hindlimb vasculature was imaged using a micro-CT imaging system (NFR-Polaris-G90; Nano Focus Ray Company, Iksan, Korea). The scanner was set to a voltage of 80 kVp and a current of 85 µA. Scans were completed over 360° of rotation of the X-ray tube. It took 700 seconds for each micro-CT scan. The reconstruction image size was 1,024 × 1,024 pixels, and the number of slices was 540.
Mice were placed in a chamber with 4% isoflurane in oxygen to induce anesthesia. During imaging, mice remained anesthetized using 1.5% isoflurane in oxygen. Mice were imaged at the baseline and then injected with a contrast agent (Fenestra VC) at a dose of 20 ml/kg via the tail vein. All micro-CT image data were acquired using live, free-breathing, anesthetized mice.
Statistical analysis. We performed statistical analysis using a paired, one-tailed Student's t-test. Statistical comparisons between experimental groups in the mouse hindlimb ischemia model were performed by Fisher's exact test. We considered data to be statistically significant when P value was <0.05.
SUPPLEMENTARY MATERIAL Figure S1. Effect of the conditioned media on growth of HUVECs. Figure S2. VEGF and FGF-2 are involved in tubule formation of HUVECs. Figure S3. Effect of the conditioned media on invasiveness of HUVECs. Figure S4. Effect of Ad-F-UCP on outgrowth of vessel-like structures in the explanted muscle. Figure S5. Verification of the mouse hindlimb ischemia. Figure S6. Primer sequences for RT-PCR.
Acknowledgments
We are grateful to S.Y. Kim, KRIBB, Korea, for statistical analysis. This research was supported by a grant (2010-0026153) from 21C Post-Frontier Functional Human Genome Program of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology of Korea, and a grant from KRIBB Research Initiative Program.
Supplementary Material
Effect of the conditioned media on growth of HUVECs.
VEGF and FGF-2 are involved in tubule formation of HUVECs.
Effect of the conditioned media on invasiveness of HUVECs.
Effect of Ad-F-UCP on outgrowth of vessel-like structures in the explanted muscle.
Verification of the mouse hindlimb ischemia.
Primer sequences for RT-PCR.
REFERENCES
- Gupta R, Tongers J., and, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA., and, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S.et al. (2003Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1 Circ Res 931074–1081. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C.et al. (2000Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor Circulation 1022255–2261. [DOI] [PubMed] [Google Scholar]
- Rey, S., and, Semenza, GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodeling. Cardiovascular Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Hwang KS, Yoo J, Cho WK, Kim JM, Kim WH.et al. (2006E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis Nat Med 12809–816. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jung CR, Lee CH., and, Im DS. Egr-1 and serum response factor are involved in growth factors- and serum-mediated induction of E2-EPF UCP expression that regulates the VHL-HIF pathway. J Cell Biochem. 2008;105:1117–1127. doi: 10.1002/jcb.21914. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ.et al. (2001Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation Science 292468–472. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME.et al. (1999The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis Nature 399271–275. [DOI] [PubMed] [Google Scholar]
- Rissanen TT., and, Ylä-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH., and, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107:2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ., and, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A., and, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y.et al. (2005VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling J Cell Sci 118Pt 163759–3768. [DOI] [PubMed] [Google Scholar]
- Jang HS, Kim HJ, Kim JM, Lee YS, Kim KL, Kim JA.et al. (2004A novel ex vivo angiogenesis assay based on electroporation-mediated delivery of naked plasmid DNA to skeletal muscle Mol Ther 9464–474. [DOI] [PubMed] [Google Scholar]
- Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K.et al. (2002Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2 Circ Res 90966–973. [DOI] [PubMed] [Google Scholar]
- Duvall CL, Taylor WR, Weiss D., and, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S.et al. (1994Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model J Clin Invest 93662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E.et al. (2003Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2 Nat Med 9604–613. [DOI] [PubMed] [Google Scholar]
- Wu S, Nishiyama N, Kano MR, Morishita Y, Miyazono K, Itaka K.et al. (2008Enhancement of angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing prolyl hydroxylase domain-2 gene Mol Ther 161227–1234. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD.et al. (1996Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1 Mol Cell Biol 164604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdak LH, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A.et al. (2000Adenovirus-mediated VEGF(121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia Circulation 102565–571. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Ye X, Doranz B., and, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Mallat Z, Tedgui A., and, Lévy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Kibel A, Gray S., and, Kaelin WG., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, Spillmann F, Meloni M, Bouby N, Richer C.et al. (2006Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems Vascul Pharmacol 45281–301. [DOI] [PubMed] [Google Scholar]
- Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi MC, TALISMAN 201 investigators et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- Niiyama, H, Huang, NF, Rollins, MD., and, Cooke, JP.2009Murine model of hindlimb ischemia J Vis Exp 23pii: 1035doi: 10.3791/1035. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of the conditioned media on growth of HUVECs.
VEGF and FGF-2 are involved in tubule formation of HUVECs.
Effect of the conditioned media on invasiveness of HUVECs.
Effect of Ad-F-UCP on outgrowth of vessel-like structures in the explanted muscle.
Verification of the mouse hindlimb ischemia.
Primer sequences for RT-PCR.