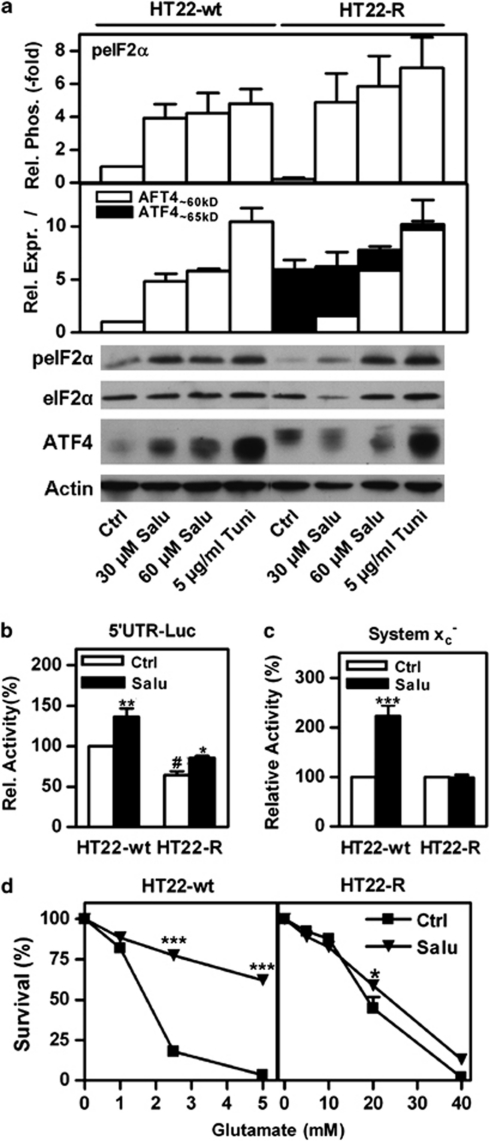
Figure 4.
The high molecular weight and ‘classical' ATF4 isoforms are inversely regulated by eIF2α phosphorylation. (a) Whole-cells extracts of HT22-wt and HT22-R cells treated with either vehicle (Ctrl) or salubrinal (Salu) at the indicated concentrations for 24 h or 5 μg/ml tunicamycin (Tuni) for 2 h were analyzed for eIF2α phosphorylation (peIF2α) relative to eIF2α expression and expression of the ‘classical' and high molecular weight ATF4 isoforms (ATF4∼60 kD and ATF4∼65 kD, respectively) relative to actin. Relative eIF2α phosphorylation and ATF4 expression were normalized to those in HT22-wt cells treated with vehicle. (b) HT22-wt and HT22-R cells were co-transfected with a luciferase construct fused to the ATF4 5′UTR and the pSV-β-GAL vector. Cells were treated either with vehicle (Ctrl) or 60 μM salubrinal (Salu). The luciferase/β-galactosidase ratio of untreated HT22-wt cells was normalized to 100%. (c and d) HT22-wt or HT22-R cells were treated with 30 μM Salubrinal (Salu) or vehicle (Ctrl) for 24 h. (c) System xc− activity was measured as HCA-sensitive sodium-independent 3H-glutamate uptake. Uptake in vehicle-treated cells was normalized to 100%. (d) Cells in 96-well plates were treated with glutamate at the indicated concentrations for 24 h in quadruplicate. Viability was measured by the MTT assay and relative survival calculated by normalizing to the MTT value of cells not treated with glutamate. The graphs show data from three (a and d) or four (b and c) independent experiments as mean±S.E.M. Statistical analysis was performed by two-way ANOVA and Bonferroni post test, *P<0.05. **P<0.01,***P<0.001 compared with control cells, #P<0.05, HT22-R cells compared with HT22-wt without salubrinal. Representative blots are shown below the graphs in (a)