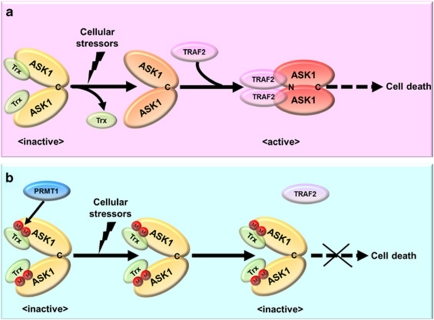
Figure 8.
A proposed model for the regulation of ASK1 by PRMT1-mediated arginine methylation. (a) In an inactive state, ASK1 associates with the reduced form of thioredoxin (Trx) through its NH2-terminal region and forms a homo-oligomer through the COOH-terminal coiled-coil domain (denoted as ‘C'). Exposure of cells to various cellular stressors including ROS induces the dissociation of thioredoxin from ASK and the recruitment of TRAF2 (or TRAF6) to ASK1. TRAF2 (or TRAF6) promotes the homophilic interaction of the NH2-terminal coiled-coil domain (denoted as ‘N') of ASK1, facilitating the formation of active ASK1 complex. Persistent activation of the ASK1 signaling leads to induction of cell death. (b) PRMT1, when its expression or activity is elevated in cells, mediates arginine methylation of ASK1. Arginine methylation of ASK1 abrogates the release of thioredoxin from ASK1 and the subsequent association between ASK1 and TRAF2, thereby inhibiting the homophilic interaction of the NH2-terminal coiled-coil domain of ASK1. Therefore, PRMT1-mediated methylation of ASK1 suppresses the activation of ASK1 and its downstream signaling processes, leading to aberrant defects of cell death