Abstract
Aging is associated with a pro-oxidant state and a decline in endothelial function. Whether acute, enteral antioxidant treatment can reverse this decrement in vascular function is not well known. Flow-mediated vasodilation and reactive hyperemia were evaluated following consumption of either placebo or an oral antioxidant cocktail (Vitamin C, 1000mg; Vitamin E, 600 I.U.; Alpha-lipoic acid, 600 mg) in 87 healthy volunteers (42 young, 25 ± 1 yrs; 45 older, 71 ± 1 yrs) using a double-blind, crossover design. Blood velocity and brachial artery diameter (ultrasound Doppler) were assessed before and after 5-min forearm circulatory arrest. Serum markers of lipid peroxidation, total antioxidant capacity, endogenous antioxidant activity, and Vitamin C were assayed, and plasma nitrate, nitrite, and 3-nitrotyrosine were determined. In the placebo trial, an age-related reduction in brachial artery vasodilation was evident (young: 7.4 ± 0.6 %; older: 5.2 ± 0.4 %). Following antioxidant consumption, flow-mediated vasodilation improved in older subjects (5.2 ± 0.4 %, placebo: 8.2 ± 0.6 %, antioxidant) but declined in the young (7.4 ± 0.6 %, placebo; 5.8 ± 0.6 %, antioxidant). Reactive hyperemia was reduced with age, but antioxidant administration did not alter the response in either group. Together, these data demonstrate that antioxidant consumption acutely restores endothelial function in the elderly, while disrupting normal endothelium-dependent vasodilation in the young, and suggest that this age-related impairment is due, at least in part, to free radicals.
Keywords: Aging, Endothelium, Free Radicals, Nitric Oxide, Vascular Function
INTRODUCTION
In recent years, considerable effort has been expended to evaluate the relationship between age, oxidative stress, and vascular health, with largely equivocal results. However, there is convincing evidence of an overall increase in plasma free radical concentration with advancing age 1, 2 and a subsequent reduction in nitric oxide (NO) bioavailability which has been implicated in the age-related decline in endothelial function 3,4–7. As endothelial dysfunction and increased vascular oxidative stress have been identified as predictors for risk of cardiovascular events 8, antioxidant (AO) supplementation as a non-invasive approach to further understand vascular function, and the potential of this approach in the prevention and treatment of vascular dysfunction, is particularly attractive in the elderly population.
Despite the accepted association between oxidative stress and vascular health, chronic, large scale (>1000 subjects) clinical trials have failed to demonstrate a beneficial effect of long-term AO consumption on cardiovascular disease morbidity and mortality 9. However, smaller, interventional studies focused on acute AO-mediated changes in vascular reactivity have provided more promising results. Infused, supra-physiologic doses of vitamin C have been documented to transiently restore endothelium-dependent vasodilation 10, 11 and skeletal muscle blood flow 12–14 in the elderly, and BH4 administration (a cofactor for endothelial NO production) acutely improves endothelial function 15. However, studies utilizing more practical interventions, such as oral vitamin C at over-the-counter doses, have failed to demonstrate an improvement in age-related endothelial dysfunction 10. Moreover, the effect of combined AOs administered concomitantly on vascular function in the elderly remains largely unknown. Thus, despite the myriad studies that have examined the role of oxidative stress on cardiovascular health with healthy aging, mechanistic studies designed to abruptly reduce plasma oxidative stress and subsequently determine the acute effects of this intervention on AO capacity, oxidative stress, and vascular function have not been undertaken.
Determination of endothelial function following an acute AO intervention is most easily achieved by assessing the degree of brachial artery vasodilation in response to an acute increase in shear stress. This flow-mediated vasodilation (FMD) of the brachial artery subsequent to a period of vascular occlusion provides a relevant index of endothelium-dependent vasodilation which reflects NO bioavailability 16 and correlates well to coronary artery reactivity 17. Importantly, both coronary 18 and peripheral 19, 20 vasodilator function have been convincingly documented to predict future cardiovascular events, and as such, assessment of endothelium-dependent vasodilation may provide valuable prognostic and diagnostic patient information. Likewise, the magnitude of reactive hyperemia relates inversely to traditional cardiovascular disease risk factors 21 and has also emerged as a clinically relevant tool in predicting future cardiovascular events. However, the role of NO in this response is minimal 22, 23, and thus reactive hyperemia remains a clinically relevant, though somewhat non-specific, indicator of microvascular reactivity. Together, FMD and reactive hyperemia may therefore be seen as complimentary measurements that allow an opportunity to compare endothelial and microvascular function, as well as determination of the specific effect of oxidative stress and AO administration on these distinct regions of the vasculature.
In the current study, we sought to fill a void in the clinical literature by administering an oral AO cocktail (vitamin C, E, and alpha-lipoic acid) to abruptly reduce plasma free radical concentration, and assess the acute vascular responses to this reduction in oxidative stress in young and older subjects. Two hypotheses were tested; 1) We hypothesized that AO consumption would improve FMD in the elderly, but decrease FMD in their young, healthy counterparts, and 2) We hypothesized that reactive hyperemia would not be affected by AO consumption in either young or older subjects.
METHODS
Subjects and General Procedures
42 young (25 ± 1 yrs) and 45 older (71 ± 1 yrs) healthy volunteers participated in the current study. All subjects were non-smokers, normotensive (<140/90 mmHg), and free of overt cardiovascular disease as determined by health history questionnaire and physical examination. Exclusion criteria included subjects with diagnosed cardiovascular disease, diabetes, hypercholesterolemia, hypertension, and women who were pregnant. Subjects were not taking any prescription medication, and were asked to abstain from vitamin supplements for 10 days prior to enrollment and during the course of the study. Subjects arrived to the laboratory at 0800 in a fasted state, having abstained from exercise and caffeine for 12 hours prior, in accordance with recently published guidelines 24. Premenopausal females were studied within the first five days of their menstrual cycle, as FMD measurements of the brachial artery have been documented to fluctuate with menstrual phase. Protocol approval and written informed consent were obtained according to the University of Utah and Veteran Affairs Institutional Review Board requirements. All data collection took place with subjects supine, in a thermoneutral environment.
Antioxidant Supplementation
All subjects reported to the laboratory twice within one week (>48 hrs apart) and received the antioxidant (AO) cocktail or placebo (PL) in a balanced, double-blind, crossover design. Supplements were taken in two doses, separated by 30 minutes to improve absorption, consumed 90 and 60 minutes prior to the FMD protocol. The first dose consisted of 300 mg of alpha-lipoic acid, 500 mg Vitamin C, and 200 I.U. Vitamin E, and the second dose was 300 mg alpha-lipoic acid, 500 mg Vitamin C, and 400 I.U. Vitamin E. Placebo microcrystalline cellulose capsules of similar taste, color, and appearance were likewise consumed in two doses within the same time frame. We have previously reported the efficacy of this antioxidant cocktail to reduce carbon- and O2-centered free radical levels, as measured by electron paramagnetic resonance (EPR) spectroscopy, in both young 25 and older 26 subject populations.
Measurements
Details of the FMD procedure have been described previously 27, and were performed in accordance with current recommendations 24. Briefly, a blood pressure cuff was placed on the right arm proximal to the elbow and distal to the placement of the ultrasound Doppler probe on the brachial artery (BA). The BA was insonated approximately midway between the antecubital and axillary regions, and measurements of BA diameter and blood velocity (Vmean) measurements were obtained continuously at rest and for 2 minutes following cuff deflation (Logiq 7, GE Medical Systems, Milwaukee, WI).
Analyses
Vmean was automatically calculated using commercially available software (Logiq 7). End-diastolic, ECG R-wave gated images were collected via video output from the Logiq 7 for off-line analysis of BA vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, Iowa). Flow-mediated vasodilation (FMD) was quantified as the maximal percent change in BA diameter following cuff release. Shear rate was calculated as: . Blood flow was calculated as: . For both shear rate and blood flow, cumulative area-under-curve (AUC) values were integrated with the trapezoidal rule and calculated as . Reactive hyperemia was quantified as cumulative BA blood flow for two minutes (AUC) following cuff occlusion. Normalized FMD was calculated by dividing FMD (%) by the cumulative shear rate AUC at the time of peak BA vasodilation.
Assays
In a subset of subjects (n = 18 young, n = 30 older), blood samples were obtained from the antecubital vein immediately prior to FMD testing on both PL and AO visits. Total AO capacity was assessed by the ferric reducing ability of plasma (FRAP) assay, and endogenous AO activity was assessed by determining superoxide dismutase (SOD, Cayman Chemical Company, Ann Arbor, MI). Plasma ascorbate concentration was also determined (Cosmo Bio, Carlsbad, CA). Quantitative determination of thiobarbituric acid reactive substances (TBARS) was performed to assess lipid peroxidation, a marker of oxidative stress (Bioassay Systems, Hayward, CA). Total nitrate and nitrite were measured using a standard colorimetric nitrate reductase/Greiss reaction assay (Cayman Chemical, Ann Arbor, MI). Measurement of nitrotyrosine (3-NT) was performed using competitive ELISA (Cell Biolabs, San Diego, CA). A lipid panel was obtained for all subjects by standard techniques.
Statistics
Statistics were performed with the use of commercially available software(SigmaPlot 11, Systat Software Inc., Point Richmond, California, USA). Repeated measures two-way analysis of variance (ANOVA) was used to identify significant changes in measured variables between conditions and groups, with the Bonferroni test used for post-hoc analysis when a significant main effect was found. All group data are expressed as mean ± standard error. Significance was established at P<0.05.
RESULTS
Subject characteristics are presented in Table 1, and assay results are documented in Table 2.
TABLE 1.
Subject Characteristics.
| Variable | Young (n = 42) | Older (n = 45) |
|---|---|---|
| Age (yrs) | 25 ± 1 | 71 ± 1* |
| Height (cm) | 172 ± 2 | 168 ± 1 |
| Weight (kg) | 64 ± 2 | 73 ± 2* |
| Body Mass Index (kg/m2) | 22 ± 1 | 25 ± 1* |
| Total cholesterol (mg/dl) | 157 ± 6 | 182 ± 8* |
| High-density lipids (mg/dl) | 55 ± 3 | 52 ± 3 |
| Low-density lipids (mg/dl) | 93 ± 4 | 114 ± 7* |
| Triglycerides (mg/dl) | 69 ± 6 | 100 ± 8 |
| BA diameter (mm) | 3.7 ± 0.1 | 4.0 ± 0.2* |
| BA blood flow (ml/min) | 44 ± 3 | 73 ± 6* |
BA, brachial artery.
Significant difference between young and older groups, P<0.05.
TABLE 2.
Serum measurements of antioxidant capacity, oxidative stress, and antioxidant activity.
| Variable | Young (n = 18) | Older (n = 30) | ||
|---|---|---|---|---|
| Condition | PL | AO | PL | AO |
| Vitamin C (μg/ml) | 1.4 ± 0.2 | 2.2 ± 0.2† | 1.9 ± 0.2 | 3.3 ± 0.3† |
| Thiobarbituric Acid Reactive Substances (TBARS, μM) | 1.51 ± 0.19 | 2.70 ± 0.13* | 2.19 ± 0.09† | |
| Ferric-reducing AO power (FRAP, μM/L) | 803 ± 32 | 947 ± 41† | 1059 ± 30* | 1194 ± 38† |
| Superoxide Dismutase (SOD, U/ml) | 9.4 ± 0.8 | 10.6 ± 0.8† | 8.3 ± 0.3 | 9.02 ± 0.3† |
| 3-Nitrotyrosine (nM) | 242 ± 21 | 275 ± 32 | 197 ± 8* | 198 ± 9* |
| Nitrite (μM) | 8.0 ± 0.7 | 8.0 ± 0.7 | 6.8 ± 1.3* | 5.3 ± 0.4* |
| Nitrite:Nitrate | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.06 ± 0.01* | 0.05 ± 0.01* |
PL, placebo; AO, antioxidant.
Significant difference between young and older groups, P<0.05;
Significant difference between PL and AO, P<0.05.
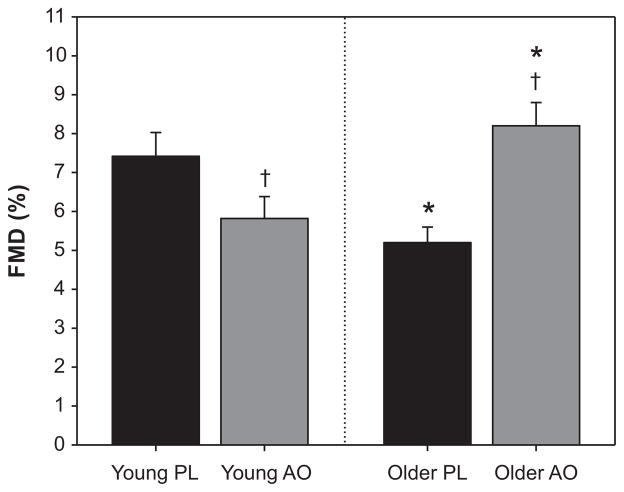
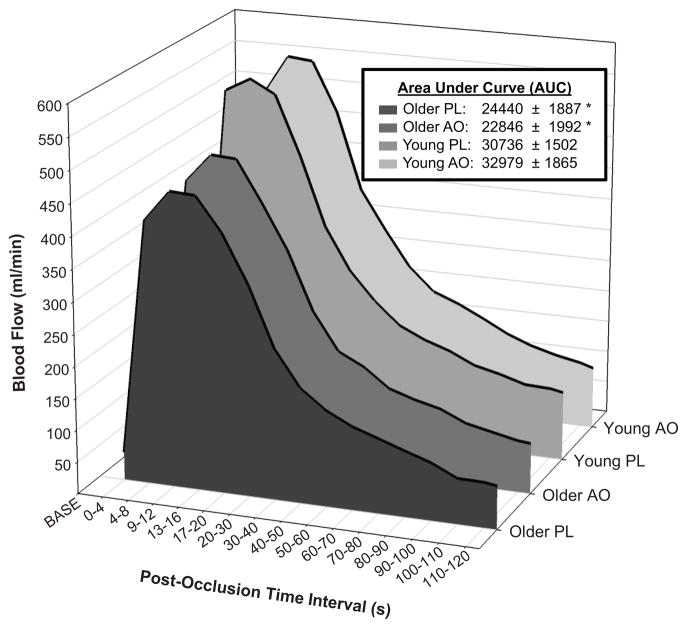
In the PL trial, FMD was significantly attenuated in older subjects compared to their younger counterparts (5.2 ± 0.4 %, older PL; 7.4 ± 0.6%, younger PL) (Figure 1). In the older group, AO administration significantly improved FMD (5.2 ± 0.4 % to 8.2 ± 0.6 %, PL vs. AO). In contrast, AO administration in the young group had a detrimental effect on FMD (7.4 ± 0.6 % to 5.8 ± 0.6 %, PL vs. AO) (Figure 1). When normalized for shear stimulus, the age-associated decrement in FMD was still evident (0.22 ± 0.03 %/s−1·s, older PL; 0.31 ± 0.03 %/s−1·s, young PL). AO administration significantly improved normalized FMD in the older group (0.22 ± 0.03 to 0.37 ± 0.03 %/s−1·s) and reduced normalized FMD in the young (0.31 ± 0.03 to 0.23 0.02 %/s−1·s, PL vs. AO). The time to peak dilation was significantly greater in the older group, but was not affected by AO administration (61 ± 3 s, older PL; 50 ± 3 s, young PL; 62 ± 4 s, older AO; 52 ± 3 s, young AO). In both the PL and antioxidant AO trials, a significant age-related decrement in reactive hyperemia was evident; however, AO treatment did not alter the hyperemic response in either group (Figure 2).
FIGURE 1.
Brachial artery flow-mediated vasodilation (FMD) in young and older subjects following placebo (PL) or antioxidant (AO) administration. In the PL trial, an age-associated decline in FMD was apparent. Following AO consumption, FMD was reduced in young, but improved in the elderly. * Significant difference between young and older groups, P<0.05; † Significant difference between PL and AO, P<0.05.
FIGURE 2.
Reactive hyperemia following 5-min cuff occlusion in young and older subjects following placebo (PL) or antioxidant (AO) administration. Elderly subjects exhibited a decline in post-occlusion blood flow, but there was no effect of AO treatment. * Significant difference between young and older groups, P<0.05. AUC, Area-under-curve.
DISCUSSION
The present study sought to evaluate the efficacy of acute AO administration to abruptly reduce plasma oxidative stress, and to subsequently determine the acute effects of this intervention on AO capacity, oxidative stress, and vascular function in young and older individuals. In the elderly, we identified a reduction in FMD that was accompanied by elevated oxidative stress, and demonstrated the ability of an acute AO intervention to reverse this age-associated decrement. In contrast, AO administration did not prove beneficial in younger individuals who had lower levels of oxidative stress, but rather resulted in a reduction in FMD. Reactive hyperemia was also lower in the elderly, but was not altered by AO in either group, supporting the NO-specific nature of the AO intervention. Together, these findings support the important role of free radicals in age-associated endothelial dysfunction, and identify the efficacy of AOs available over the counter as capable of acutely improving endothelial function in the elderly.
FMD, Age, and Antioxidants
Brachial artery FMD testing has emerged as a simple, non-invasive means of evaluating endothelial function in a variety of healthy and disease populations 24, 28, 29. Vasodilation of the brachial artery subsequent to a period of vascular occlusion provides an index of endothelium-dependent vasodilation which reflects NO bioavailability 16 and is thought to be predictive of future cardiovascular events 19, 20. These previous studies have led to the concept that results of endothelial function testing may serve as a “barometer for cardiovascular health” 30 and promoted interest in interventions with the prospect of improving endothelial health and thereby limiting cardiovascular risk.
We observed an age-related decline in FMD in the elderly (Figure 1), which is in agreement with the majority of studies that have collectively identified a decline in endothelium-dependent vasodilation with advancing age, often as early as the fourth decade 31–33. However, this age-related decrement appeared readily reversible, as evidenced by improved FMD following AO administration. Indeed, AO consumption acutely improved brachial artery FMD in the elderly to that of their younger counterparts (Figure 1), suggesting that an AO cocktail at modest, enteral doses is sufficient to acutely reverse age-related endothelial dysfunction. This profound improvement in endothelial function extends former studies demonstrating the capacity of high-dose, infused AOs to acutely improve endothelium-dependent vasodilation 4, 10, 15 in the elderly, now revealing a similar beneficial effect using a typical, over-the-counter combination of AOs available to the general public. As discussed above, FMD is generally thought to provide a bioassay of NO 16, and as such it is likely that this AO-mediated improvement in FMD in the elderly was achieved through an acute increase in NO bioavailability.
In contrast to the positive outcome in the elderly, the AO intervention proved detrimental to endothelium-dependent vasodilation in young, healthy individuals (Figure 1). Previous studies from our group have identified a similar pattern of “paradoxical” age-specific AO effects, which appears to be related to the underlying level of oxidative stress 34. Specifically, during handgrip exercise, BA vasodilation is impaired in the elderly, but this age effect is restored to that of the young following AO consumption 35; however, AO administration had an opposite effect in the young, resulting in an impaired vasodilatory capacity during exercise 25. Findings from the present study thus complement these previous exercise studies through the use of BA FMD measurements, a widely accepted method for examining endothelial function 28.
Evidence of negative responses to AO in the young appears to support the emerging concept of a beneficial role for free radicals in the peripheral circulation. Indeed, others have documented that free radicals such as H2O2 and reactive oxygen species (i.e. ONOO−) may also act as potent vasodilators 36 and thus may, in fact, contribute to FMD37. In addition to direct vasodilatory properties, free radicals may also play an important role in upregulation of endogenous AO capacity though increased expression of several enzymes known to “detoxify” free radicals 38. Considering these former studies and the present data, we speculate that in the young, where pro and antioxidant forces are somewhat balanced, the aggressive reduction in free radical concentration following AO administration may have removed or suppressed certain oxidative species which possess some beneficial properties in terms of vascular regulation, resulting in the observed decrement in FMD.
Together, these data in young and older individuals demonstrate a dichotomous effect of AO consumption on endothelial function with age; consumption of an AO cocktail at over-the-counter doses acutely improved endothelial function in the elderly, while disrupting normal endothelium-dependent vasodilation in the young.
Reactive Hyperemia, Age, and Antioxidants
Following circulatory occlusion, resistance vessel vasodilation provokes an increase in arm vascular conductance, and thus acts as the downstream stimulus for upstream increases in BA shear rate and subsequent BA vasodilation. However, the factors which contribute to reactive hyperemia per se are thought to be somewhat more complex than BA FMD. Indeed, this response may be attributed to a combination of endothelium-dependent dilation, inherent myogenic action, and vasodilatory metabolites produced in response to tissue ischemia, including adenosine and non-endothelium dependent vasodilators.
Like FMD, the magnitude of reactive hyperemia relates inversely to traditional cardiovascular disease risk factors 21 and has thus emerged as a clinically relevant tool in predicting future cardiovascular events39. However, the role of NO in this response appears modest 22, 23, and thus reactive hyperemia remains a clinically relevant, though somewhat non-specific, indicator of microvascular reactivity. In the present study, these complimentary measurements allowed inter- and intra-subject comparison of the effects of AO administration on both endothelial and microvascular function, as well as an opportunity to further define the specific effect of acute AO consumptions on NO bioavailability. As illustrated in Figure 2, reactive hyperemia was significantly reduced in older subjects in the PL trial. The mechanisms for this age-related difference remain unknown, but it is speculated that an age-related decline in sensitivity to ischemia or production of vasodilatory metabolites may at least partially explain these intriguing findings. This supposition is supported by the recent observation that the muscle metaboreflex becomes less sensitive with advancing age 40. AO administration did not alter reactive hyperemia in either group, which was anticipated based on former work from our group 27 and others 22 which failed to identify NO as a significant contributor to post-occlusion reactive hyperemia. These findings are in line with other AO studies which have been unable to document a change in reactive hyperemia following intra-arterial ascorbic acid 10 or BH4 15 in elderly subjects. When viewed in conjunction with the marked AO effect on FMD, the lack of a treatment effect in the microvasculature further supports the assertion that the AO cocktail consumed in the present study likely improved endothelial function in the elderly through a reduction in oxidative stress and subsequent improvement in conduit artery NO bioavailability. It is also tempting to speculate that free radicals may play a proportionally bigger role than NO in governing reactive hyperemia through vascular smooth muscle hyperpolarization or the oxidative activation of the cGMP-dependent kinase via disulfide-dimerization, though discerning these mechanistic pathways is beyond the scope of the present study.
Quantitative Assessment of Antioxidant Efficacy
One essential component of any study examining the efficacy of an AO intervention is detecting increases in circulating AO concentrations and documenting a subsequent reduction in free radical concentration and NO production. In terms of AO, we determined that vitamin C concentration and markers of AO capacity increased in both young and older groups (Table 2), confirming the equally efficacious effect of the AO treatment between groups. It is thus apparent that AO-mediated changes in endothelial function cannot be attributed to differences in absorption of the AO cocktail. It is also noteworthy that administration of exogenous AOs increased superoxide dismutase (SOD) activity (Table 2), one of the most ubiquitous endogenous AOs. It thus seems that AO consumption resulted in a two-tier effect, including both direct scavenging effects of free radicals and a secondary decrease in oxidative stress via “sparing” of endogenous AOs, which collectively improved endothelial function in the elderly.
Oxidative stress was significantly reduced following AO administration in the elderly, as documented by a reduction in lipid peroxidation end-products (TBARS) (Table 2). Interestingly, the same cannot be said for the young, in whom TBARS did not decrease as a consequence of the AO cocktail. In combination with our previous reports identifying the efficacy of this AO cocktail to reduce carbon- and O2-centered free radical levels utilizing EPR spectroscopy in both young 25 and older 26 subject populations, confirms the ability of this AO treatment to acutely reduce oxidative stress when baseline levels are elevated, as in the elderly. Surprisingly, AO consumption did not affect 3-Nitrotyrosine in either group, and was reduced in the elderly compared to young in the placebo condition (Table 2). This was an unexpected finding, considering the age-related increase in TBARS and the subsequent reduction following AO consumption in the older group, but is in agreement with prior work reporting similar tissue 3-Nitrotyrosine levels in both young and older rats before and after chronic AO supplementation 41.
Plasma Nitrite and Nitrite:Nitrate were assessed as a surrogate measure of vascular NO bioavailability (Table 2), as it has been demonstrated that circulating nitrite sensitively reflects acute changes in regional eNOS activity 42. For both Nitrite and Nitrite:Nitrate, we observed an age-related decline that is consistent with previous studies 7 and the functional measurements of FMD (Figure 2). However, we were unable to detect a significant decrease in either Nitrite or Nitrite:Nitrate following AO consumption. As with the 3- Nitrotyrosine, this lack of an AO effect on plasma Nitrite and Nitrite:Nitrate may reflect the somewhat subtle nature of the oral AO treatment utilized in the present study, an intervention that appears to provoke functional improvements in endothelium-dependent vasodilation without detectable increases in circulating nitrite levels.
Perspectives
In contrast to the largely disappointing findings from clinical trials concerning the beneficial effect of chronic AO administration on cardiovascular health 9, the present study has identified a marked and acute improvement in endothelial-dependent vasodilation in the elderly following consumption of an oral AO cocktail. However, the difference in design between acute and long-term interventional studies precludes a direct comparison with data from these previous studies. Indeed, the present study administered a single AO load in order to provoke an abrupt reduction in plasma oxidative stress, and subsequently determine the acute effects of this intervention on AO capacity, oxidative stress, and vascular function in young and older individuals. Employing this short-term, interventional design has allowed us to demonstrate the ability of an oral AO cocktail to reverse the age-associated impairment in endothelial function in the elderly without the additional confounding variables associated with longer-term treatment studies. These findings provide important mechanistic insight regarding the link between endothelial dysfunction and oxidative stress in the elderly, and demonstrate a striking plasticity in endothelial function in response to a simple, oral AO intervention.
Experimental Considerations
It should be noted that the 3-Nitrotyrosine assay reflects peroxynitrite from multiple upstream sources, and is the combined consequence of basal NO bioavailability, superoxide levels, and SOD activity 43 . Thus, the reduction in 3-Nitrotyrosine in the elderly, and lack of an effect following AO administration, is somewhat difficult to interpret due to this assay’s lack of specificity. We also acknowledge that the present data does not offer insight concerning the potential vascular benefits of a long-term AO treatment. It is anticipated that follow-up studies involving chronic administration of this oral AO cocktail will build upon these acute vascular measurements, allowing a more appropriate comparison to existing, larger-scale AO studies.
Summary
Administration of an AO cocktail documented to reduce plasma oxidative stress significantly improved endothelial function in the elderly, reversing the age-related decrement in FMD. In contrast, the same AO intervention proved detrimental to endothelium-dependent vasodilation in young, healthy individuals. AO administration did not alter reactive hyperemia in either group, supportive of an endothelium-dependent, NO-specific effect. These findings reveal a pivotal role of oxidative stress in age-associated endothelial dysfunction, and support the efficacy of oral AO administration in physiologic doses as a means to acutely improve conduit artery endothelial function in the elderly.
NOVELTY AND SIGNIFICANCE.
What is New?
A single dose of antioxidant vitamins C, E and alpha-lipoic acid improved endothelium-dependent vasodilation in the elderly to a level comparable to that of their younger, healthy counterparts. This new finding suggests that acute antioxidant consumption at physiologic doses is sufficient to acutely reverse age-related endothelial dysfunction.
In contrast to the positive outcome in the elderly, the AO intervention proved detrimental to endothelium-dependent vasodilation in young, healthy individuals. This “paradoxical” effect of AO on vascular function appears to be related to differences in the underlying level of oxidative stress between young and older populations.
What is Relevant?
In contrast to the largely disappointing findings from clinical trials concerning the beneficial effect of chronic AO administration on cardiovascular health, the present study has identified a marked and acute improvement in endothelial-dependent vasodilation in the elderly following consumption of an oral AO cocktail. As oxidative stress and endothelial function commonly accompany geriatric hypertension, these findings in the elderly may represent a potential therapeutic target for improving disease-related decrements in endothelium-dependent vasodilation.
Summary
Findings from the present study have identified a pivotal role of oxidative stress in age-associated endothelial dysfunction, and support the efficacy of oral AO administration in physiologic doses as a means to acutely improve conduit artery endothelial function in the elderly.
Acknowledgments
Funding Sources: Funded in part by NIH PO1 HL-091830 (R.S.R.), TRDRP 15RT-0100 (R.S.R.), VA RR&D E6910R (R.S.R.), AHA 0835209N (D.W.W), and AHA 10SDG305006 (R.A.H).
Footnotes
Financial Disclosures: None.
References
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhoutte PM. Ageing and endothelial dysfunction. Eur Heart J Supplements. 2002;49:A8–A17. [Google Scholar]
- 4.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 5.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan A, More RS, Mullins PA, Taylor G, Petch C, Schofield PM. Aging-associated endothelial dysfunction in humans is reversed by l-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 7.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 9.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 10.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of no availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 12.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 13.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide but not vasodilating prostaglandins contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:H1633–441. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: Impact of acute ascorbic acid administration. The Journal of physiology. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: The contribution of nitric oxide. Clinical science. 2001;101:629–635. [PubMed] [Google Scholar]
- 17.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 18.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 19.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 21.Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide–mediated shear stress– and agonist-induced dilations. Circ Res. 1998;83:960–965. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- 22.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation. 1994;90:2285–2290. doi: 10.1161/01.cir.90.5.2285. [DOI] [PubMed] [Google Scholar]
- 23.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 24.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow- mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: Role of free radicals. Am J Physiol Heart Circ Physiol. 2007;292:H1516–1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 26.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: A radically different outcome. Clin Sci (Lond) 2009;116:433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 27.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol. 2009;107:445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 28.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 29.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 30.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 31.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The framingham heart study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 33.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 34.Wray DW, Nishiyama SK, Donato AJ, Carlier P, Bailey DM, Uberoi A, Richardson RS. The paradox of oxidative stress and exercise with advancing age. Exerc Sport Sci Rev. 2011;39:68–76. doi: 10.1097/JES.0b013e31820d7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: Effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298:H671–678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of h2o2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circulation research. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 38.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 40.Markel TA, Daley JC, 3rd, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation. 2003;107:675–678. doi: 10.1161/01.cir.0000055190.81716.ab. [DOI] [PubMed] [Google Scholar]
- 41.Leeuwenburgh C, Hansen P, Shaish A, Holloszy JO, Heinecke JW. Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. The American journal of physiology. 1998;274:R453–461. doi: 10.1152/ajpregu.1998.274.2.R453. [DOI] [PubMed] [Google Scholar]
- 42.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 43.Halliwell B, Zhao K, Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: A personal view of recent controversies. Free Radic Res. 1999;31:651–669. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]