Figure 3.
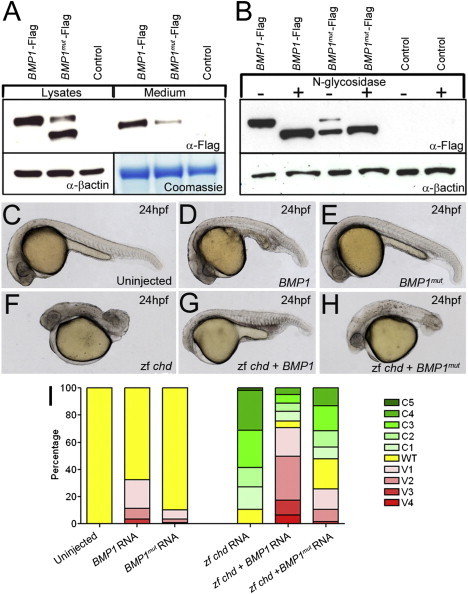
A Signal-Peptide Substitution in BMP1 Causes Secretion and Glycosylation Defects In Vitro and Loss of Protease Activity In Vivo
(A) Immunoblot of HEK 293T cells transfected with either Flag-tagged WT BMP1 (BMP1-Flag; lanes 1 and 4) or p.Gly12Arg-substituted BMP1 (BMP1mut-Flag; lanes 2 and 5) and untransfected control cells (control; lanes 3 and 6). Immunoblotting shows that the p.Gly12Arg protein (lanes 1–3) isolated from cell lysates had increased mobility; reduced amounts of this BMP1 protein were secreted into the medium (lanes 4–6).
(B) Immunoblot of lysates of HEK 293T cells transfected with either Flag-tagged WT BMP1 (BMP1-Flag; lanes 1 and 2) or p.Gly12Arg-substituted BMP1 (BMP1mut-Flag; lanes 3 and 4) and untransfected controls (control; lanes 5 and 6). After being harvested, lysates were either treated with N-glycosidase (lanes 2, 4, and 6) or left untreated (lanes 1, 3, and 5). The predominant mutant-BMP1 band with increased mobility migrates at the same rate as deglycosylated WT BMP1.
(C–I) The p.Gly12Arg-substituted BMP1 exhibits reduced Chordinase activity in vivo. Lateral views of uninjected 24 hpf zebrafish embryos (C) and embryos injected with RNA encoding either WT BMP1 (BMP1; D and G) or p.Gly12Arg signal-peptide variant BMP1 (BMP1mut; E and H). Chordinase activity was assessed by its ability to ventralize WT embryos (C–E) or rescue dorsalized (chordin RNA injected) embryos (F–H). In both assays, the mutant BMP1 showed reduced ability to counteract either the endogenous or exogenous Chordin (quantified in I).
