Abstract
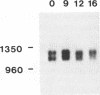
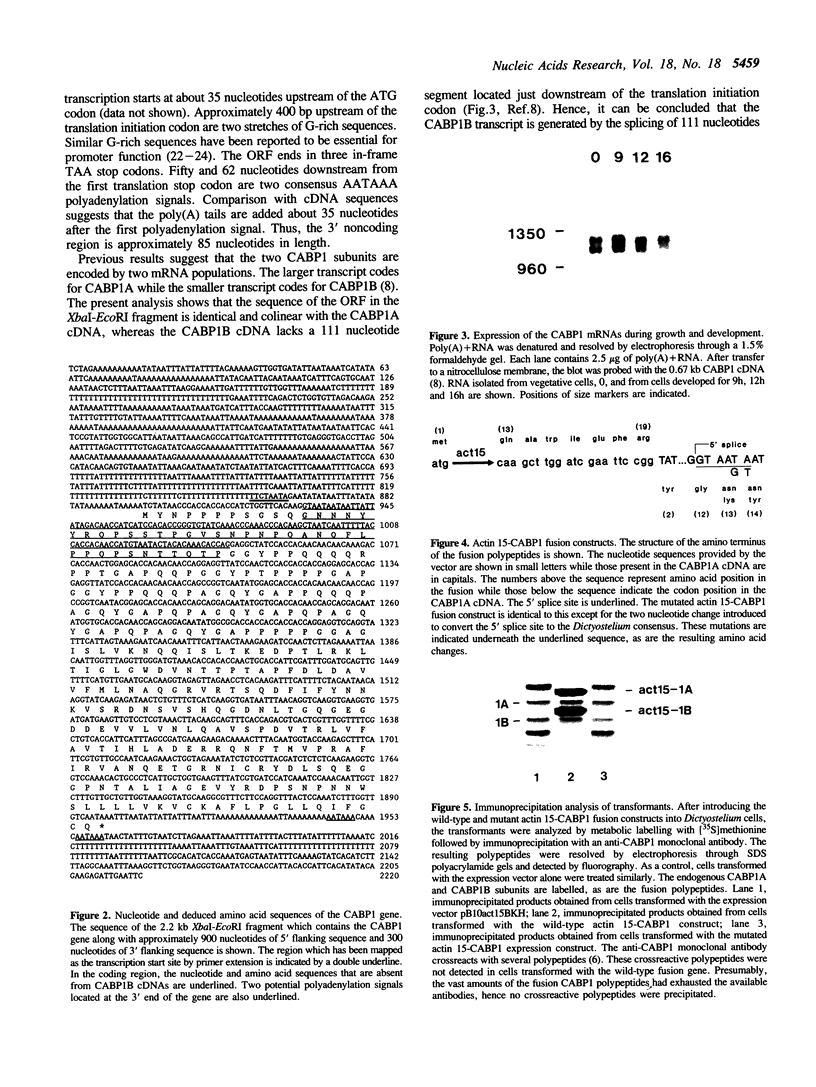
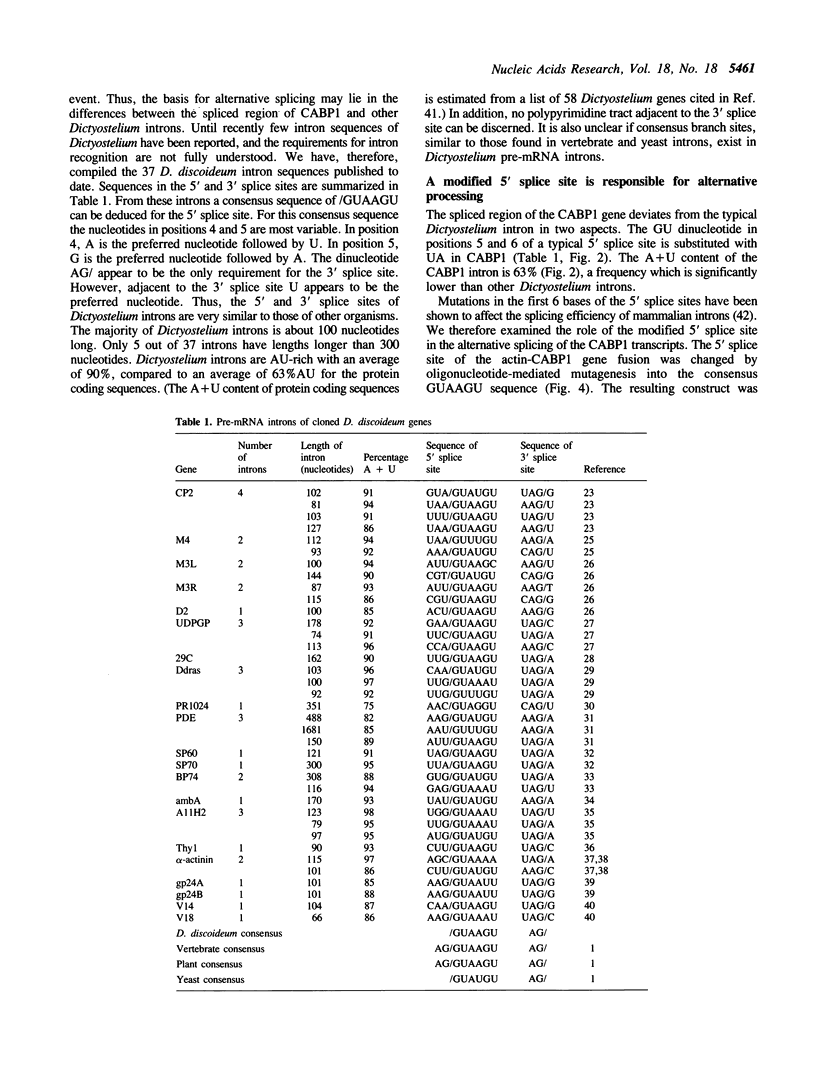
We have determined the nucleotide sequence of the CABP1 gene from Dictyostelium discoideum. Together with previous data on cDNA sequences, we establish that alternative splicing of transcripts derived from this gene is responsible for the production of the two CABP1 subunits. RNA blot analysis suggested that alternative splicing of the CABP1 transcripts occurs during growth and throughout development. In addition, we have compiled the intron sequences of Dictyostelium pre-mRNAs and observed that the GUAAGU hexanucleotide at the 5' splice site is highly conserved. The 5' splice site of CABP1 deviates from the consensus hexanucleotide in having a sequence of GUAAUA. To assess the role of the modified 5' splice on differential splicing, we have constructed an actin-CABP1 fusion gene and transformed it into Dictyostelium cells. Analysis by immunoprecipitation, with anti-CABP1 antibody and amplification of specific cDNAs by polymerase chain reaction show that the transcripts generated by the fusion gene are alternatively spliced. When the 5' splice site of the fusion gene is mutated to conform to the consensus sequence, the resulting transcripts are constitutively spliced. These observations suggest that changes in positions 5 and 6 of the donor splice site are involved in the alternative splicing of the CABP1 transcripts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Ayres K., Neuman W., Rowekamp W. G., Chung S. Developmental regulation of DNase I-hypersensitive sites in Dictyostelium discoideum. Mol Cell Biol. 1987 May;7(5):1823–1829. doi: 10.1128/mcb.7.5.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Williams K. L., Williams J. G., Ceccarelli A. Complementation of a Dictyostelium discoideum thymidylate synthase mutation with the mouse gene provides a new selectable marker for transformation. Nucleic Acids Res. 1989 May 25;17(10):3655–3661. doi: 10.1093/nar/17.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., Firtel R. A. An 80-bp cis-acting regulatory region controls cAMP and development regulation of a prestalk gene in Dictyostelium. Genes Dev. 1988 Mar;2(3):294–304. doi: 10.1101/gad.2.3.294. [DOI] [PubMed] [Google Scholar]
- Datta S., Firtel R. A. Identification of the sequences controlling cyclic AMP regulation and cell-type-specific expression of a prestalk-specific gene in Dictyostelium discoideum. Mol Cell Biol. 1987 Jan;7(1):149–159. doi: 10.1128/mcb.7.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynes J. L., Firtel R. A. Molecular complementation of a genetic marker in Dictyostelium using a genomic DNA library. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7966–7970. doi: 10.1073/pnas.86.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early A. E., Williams J. G. Two vectors which facilitate gene manipulation and a simplified transformation procedure for Dictyostelium discoideum. Gene. 1987;59(1):99–106. doi: 10.1016/0378-1119(87)90270-8. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K. L., Loomis W. F. Spore coat genes SP60 and SP70 of Dictyostelium discoideum. Mol Cell Biol. 1989 Nov;9(11):5215–5218. doi: 10.1128/mcb.9.11.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouser L. A., Friesen J. D. Mutations in a yeast intron demonstrate the importance of specific conserved nucleotides for the two stages of nuclear mRNA splicing. Cell. 1986 Apr 11;45(1):81–93. doi: 10.1016/0092-8674(86)90540-4. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Hopkinson S. B., Pollenz R. S., Drummond I., Chisholm R. L. Expression and organization of BP74, a cyclic AMP-regulated gene expressed during Dictyostelium discoideum development. Mol Cell Biol. 1989 Oct;9(10):4170–4178. doi: 10.1128/mcb.9.10.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A., Rodriguez J. R., Rosbash M. A quantitative analysis of the effects of 5' junction and TACTAAC box mutants and mutant combinations on yeast mRNA splicing. Cell. 1985 Dec;43(2 Pt 1):423–430. doi: 10.1016/0092-8674(85)90172-2. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989 May;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Intervening sequences in a Dictyostelium gene that encodes a low abundance class mRNA. Nucleic Acids Res. 1980 Dec 11;8(23):5599–5610. doi: 10.1093/nar/8.23.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon A., Scott M. P. Sequence of a Drosophila segmentation gene: protein structure homology with DNA-binding proteins. Nature. 1984 Jul 5;310(5972):25–31. doi: 10.1038/310025a0. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Fuller D. L. A pair of tandemly repeated genes code for gp24, a putative adhesion protein of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1990 Feb;87(3):886–890. doi: 10.1073/pnas.87.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. Cyclic AMP regulation of early gene expression in Dictyostelium discoideum: mediation via the cell surface cyclic AMP receptor. Mol Cell Biol. 1987 Jan;7(1):458–469. doi: 10.1128/mcb.7.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Westphal M., Noegel A., Gerisch G. A developmentally regulated gene product from Dictyostelium discoideum shows high homology to human alpha-L-fucosidase. FEBS Lett. 1989 Mar 27;246(1-2):185–192. doi: 10.1016/0014-5793(89)80280-7. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Lin R. J., Cheng S. C., Abelson J. Molecular consequences of specific intron mutations on yeast mRNA splicing in vivo and in vitro. Cell. 1985 Aug;42(1):335–344. doi: 10.1016/s0092-8674(85)80129-x. [DOI] [PubMed] [Google Scholar]
- Noegel A., Witke W., Schleicher M. Calcium-sensitive non-muscle alpha-actinin contains EF-hand structures and highly conserved regions. FEBS Lett. 1987 Sep 14;221(2):391–396. doi: 10.1016/0014-5793(87)80962-6. [DOI] [PubMed] [Google Scholar]
- Parker R., Guthrie C. A point mutation in the conserved hexanucleotide at a yeast 5' splice junction uncouples recognition, cleavage, and ligation. Cell. 1985 May;41(1):107–118. doi: 10.1016/0092-8674(85)90065-0. [DOI] [PubMed] [Google Scholar]
- Pears C. J., Williams J. G. Multiple copies of a G-rich element upstream of a cAMP-inducible Dictyostelium gene are necessary but not sufficient for efficient gene expression. Nucleic Acids Res. 1988 Sep 12;16(17):8467–8486. doi: 10.1093/nar/16.17.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski G. J., Franke J., Faure M., Kessin R. H. The cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum utilizes alternate promoters and splicing for the synthesis of multiple mRNAs. Mol Cell Biol. 1989 Sep;9(9):3938–3950. doi: 10.1128/mcb.9.9.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragheb J. A., Dottin R. P. Structure and sequence of a UDP glucose pyrophosphorylase gene of Dictyostelium discoideum. Nucleic Acids Res. 1987 May 11;15(9):3891–3906. doi: 10.1093/nar/15.9.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Devine K. M. Codon usage and gene expression level in Dictyostelium discoideum: highly expressed genes do 'prefer' optimal codons. Nucleic Acids Res. 1989 Jul 11;17(13):5029–5039. doi: 10.1093/nar/17.13.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Manning S. S., Ken R. Primary structure and regulation of vegetative specific genes of Dictyostelium discoideum. Nucleic Acids Res. 1989 Dec 11;17(23):9679–9692. doi: 10.1093/nar/17.23.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel L. F., Smyth A., Jacobson A. Nucleotide sequence and characterization of the transcript of a Dictyostelium ribosomal protein gene. Nucleic Acids Res. 1987 Dec 23;15(24):10285–10298. doi: 10.1093/nar/15.24.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus M. A., Warrick H. M., Spudich J. A. Multiple actin-based motor genes in Dictyostelium. Cell Regul. 1989 Nov;1(1):55–63. doi: 10.1091/mbc.1.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. S., Kay C. A., Tasaka M. Expression of an altered cAMP binding protein by rapid-developing strains of Dictyostelium discoideum. Dev Biol. 1987 Mar;120(1):294–298. doi: 10.1016/0012-1606(87)90126-6. [DOI] [PubMed] [Google Scholar]
- Tsang A. S., Tasaka M. Identification of multiple cyclic AMP-binding proteins in developing Dictyostelium discoideum cells. J Biol Chem. 1986 Aug 15;261(23):10753–10759. [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Herrero J. J., Filipowicz W. Nuclear pre-mRNA processing in plants: distinct modes of 3'-splice-site selection in plants and animals. Mol Cell Biol. 1988 May;8(5):2042–2051. doi: 10.1128/mcb.8.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Witke W., Noegel A. A. A single base exchange in an intron of the Dictyostelium discoideum alpha-actinin gene inhibits correct splicing of the RNA but allows transport to the cytoplasm and translation. J Biol Chem. 1990 Jan 5;265(1):34–39. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–350. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]
- van Santen V. L., Spritz R. A. Splicing of plant pre-mRNAs in animal systems and vice versa. Gene. 1987;56(2-3):253–265. doi: 10.1016/0378-1119(87)90142-9. [DOI] [PubMed] [Google Scholar]