Figure 1.
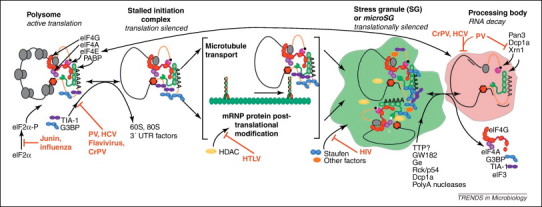
Stress granules (SGs) are intermediate compartments in mRNA metabolism. Inhibition of translation initiation leads to the disassembly of polysomes and the formation of stalled 48S initiation complexes. These messenger ribonucleoprotein (mRNP) complexes are recognized via an unknown mechanism and are remodeled, marking them for inclusion in SGs despite continued association with pro-translation initiation factors. SG components such as RasGAP SH3-domain binding protein 1 (G3BP1), Fragile X mental retardation protein (FMRP) and others are post-translationally modified, and small dispersed aggregates of remodeled mRNP complexes are transported by microtubule-associated motor proteins into larger SGs. The brackets around this central step indicate that it is not currently known which process is initially undertaken. SGs are thought to be sites of storage of stabilized mRNA, although it is known that mRNA can be released for translation or transported to processing bodies (PBs) for active decay by an unknown mechanism. Multiple virus systems (in red) have been found to interfere with the process of SG and PB formation and the points of interaction with the process are indicated. Stress granules also dock with PBs where mRNP modification and cargo exchange takes place. Initiation factors are lost except eukaryotic translation initiation factor (eIF4E) and deadenylase complexes (Pan2/3, Caf1/Ccr4) decapping complexes (Dcp1a/2) and exonucleases (Xrn1) become associated. Some viruses inhibit PB formation as indicated and poliovirus (PV) antagonizes specific PB components 43, 45, 71.
