Abstract
Delayed onset of cardiovascular disease among females is not well understood, but could be in part due to the protective effect of estrogen before menopause. Experimental studies have identified the angiotensin type 1 receptor (AT1R) as a key factor in the progression of CVD. In this study, we examined the effects of the estrogen metabolite, 2-methoxyestradiol (2ME2), on AT1R expression. Rat liver cells were exposed to 2ME2 for 24 h and angiotensin II (AngII) binding and AT1R mRNA expressions were assessed. In the presence of 2ME2, cells exhibited significant down-regulation of AngII binding in a dose and time dependent manner, independent of estrogen receptors (ERα/ERβ). Down-regulation of AngII binding was AT1R specific with no change in receptor affinity. Under similar conditions, we observed lower expression of AT1R mRNA, significant inhibition of AngII mediated increase in intracellular Ca2+, and increased phosphorylation of ERK1/2. Pretreatment of cells with the MEK inhibitor PD98059 prevented 2ME2 induced ERK1/2 phosphorylation and down-regulation of AT1R expression, suggesting that the observed inhibitory effect is mediated through ERK1/2 signaling intermediate(s). Similar analyses in stably transfected CHO cell lines with a constitutively active cytomegalovirus (CMV) promoter showed no change in AT1R expression suggesting that 2ME2 mediated effects are through transcriptional regulation. The effect of 2ME2 on AT1R down-regulation through ERK1/2 were consistently reproduced in primary rat aortic smooth muscle cells. As AT1R plays a critical role in the control of cardiovascular diseases, 2ME2-induced changes in receptor expression may provide beneficial effects to the cardiovascular as well as other systems.
Keywords: Cardiovascular disease, 2-methoxyestradiol (2ME2), hypertension, MAP- kinase
Introduction
The renin-angiotensin system functions through endocrine and paracrine/autocrine signaling, most notably in the heart, brain, kidney, and vasculature [1,2]. Circulating and locally produced angiotensin II (AngII) controls a diverse array of processes, including the maintenance of blood pressure and fluid and electrolyte balance, regulation of cell growth and neuromodulatory functions [3]. Angiotensin II binds to at least two known high-affinity receptor subtypes termed angiotensin type 1 (AT1R) and angiotensin type 2 (AT2R) that mediate angiotensin II functions through signal transduction [4]. Although both receptors have high affinities for AngII, they dictate distinct cellular functions [5]. AT1R is the major subtype expressed in adults [6]. Therefore, most pathologic cardiovascular and inflammatory effects of AngII are mediated through the AT1R. Previous studies, including clinical trials, have already demonstrated that AT1R blockade results in pleiotropic effects preventing or delaying cardiovascular events, new-onset diabetes, and renal disease, all providing long-term clinical benefits [7]. Therefore, agents that affect the responsiveness of tissues to AngII are of particular interest to researchers, especially with respect to AT1R.
Trends in epidemiological studies have suggested that there is a correlation with gender and cardiovascular morbidity and mortality, which is believed to be attributable to cardioprotective actions of select female hormones [8,9]. Nevertheless, two clinical trials involving the direct supplementation of estradiol, the Women’s Health Initiative (WHI) and the Heart and Estrogen/Progestin Replacement Study (HERS), were abandoned due to their increased number of cardiovascular events and failure to reduce cardiovascular risks [10]. Estradiol, like all sex steroids, is derived from cholesterol. Cholesterol is first converted to androstendione, then either to testosterone which then undergoes aromatization to form estradiol or, in an alternative pathway, androstedione is aromatized to estrone which is then converted to estradiol, all via an intricate network of Cytochrome p450 enzymes and hydroxysteroid dehydrogenases [11]. A comprehensive understanding of the mechanics of estradiol production is essential, as its subsequent metabolism results in the agent of interest for the current study. During endogenous metabolism, estradiol is hydroxylated to 2-hydroxyestradiol and 4-hydroxyestradiol by Cytochrome 450 enzymes. 2-hydroxyestradiol is then converted to one of its mono-methoxy estradiol metabolites, 2-methoxyestradiol (2ME2) by COMT (catechol-O-methyl transferase) [12]. The relative quantities of this estradiol metabolite vary according to gender differences as well as hormonal fluctuations during menses. In males, 2ME2 median levels are below 10 pg/ml, whereas levels in females may vary anywhere from 46 pg/ml to 70 pg/ml, excluding pregnancy in which levels may increase to over 3,500 pg/ml [13]. In postmenopause, the levels of 2ME2 drop to a median concentration of 33 pg/ml, with very little fluctuation reflecting static estradiol titers [13]. However, plasma concentrations of this metabolite postmenopause reflect a reduction of circulating estrogen and estrogen metabolites, though studies have shown that certain tissue concentrations may be significantly higher than in the plasma [14], and thus concentration dependent effects are difficult to interpret in vivo.
Estradiol can exert its action by diverse mechanisms. Classically, estradiol binds estrogen receptors (ER) and results in regulated transcription [15]. The activated estrogen receptors may then bind to Estrogen Response Elements (EREs) and attract co-activators or co-repressors to target genes that regulate transcription [16]. There are additional mechanisms by which ERs may affect cellular response. The receptor may be tethered to transcription factor complexes through protein-protein interactions affecting genes that lack EREs [17,18]; additionally, activation of growth factor receptor pathways and consequently mitogen activated protein (MAP) kinases may directly phosphorylate ERs in the absence the typical ligand [19,20]. Interestingly, estradiol is capable of directly activating intracellular kinase pathways affecting downstream transcription factors such as AP-1 [21] through MAP kinase activation, or NF-κB by way of the PI3-kinase/Akt signaling pathway [22], each initiated by 17β-estradiol. MAP kinases are serine/threonine kinases involved in cell growth, differentiation, apoptosis, etc… [23]. Within the encompassing term MAP Kinase there are three parallel cascades, each with a distinct subset of cellular consequences; these three parallel pathways are the extracellular signal regulated kinase (ERK) pathway, the stress activated protein kinase (SAPK or JNK for c-Jun-N-terminal kinase) pathway, and the p38/MAP kinase pathway [24,25]. It has been shown that estrogens activate ERK1/2 (or MAP Kinase p42/p44) in certain human breast cancer cell lines and also in cardiomyocytes [24–27]. However the mechanisms by which 2ME2 exerts its effects are largely unknown. Interestingly, epidemiological studies suggest that HRT results in improved prognosis of cardiovascular complications [28,29]. In focused studies, estradiol or 2ME2 supplementation has shown specific protective effects against atherosclerosis [30], multiple myeloma [31,32], rheumatoid arthritis and osteoporosis [33], and multiple sclerosis [34,35].
Though the mechanism by which 2ME2 exerts its effects remains unconfirmed, studies have shown that estradiol metabolites are capable of affecting the cardiovascular system, often in a beneficial manner. Specifically, it has been found that the metabolites 2-hydroxyestradiol and 2ME2 inhibit endothelin synthesis, a vaso-occlusive potentiator [36]. 17β-estradiol and the same two metabolites also have been shown to inhibit cardiac fibroblast and aortic smooth muscle cell growth in a manner that appears to antagonize ERK1/2 activation [37,38]. In addition, the anti-proliferative effects of 2ME2 have been shown to be independent of ER activation, suggesting an entirely novel action induced by estradiol and its metabolites [39]. Investigators have attempted to determine the genes responsible for 2ME2’s vasoprotective effects, and while many have been thought to be of significant importance, a single vasoprotective mediator or gene axis has yet to be identified [40].
In this study, we examined the role of synthetic 2ME2 in the regulation of AT1R. The focus of this study was to investigate the effect of 2ME2 on AT1R expression and determine the mechanisms responsible for its unique regulatory properties. We proposed that 2ME2 plays a pivotal role in the regulation of AT1R expression and function. The long-term goal of the continuing study is to assess the therapeutic potential of this naturally low-level metabolite in modifying AT1R expression and enhancing cardiovascular protection. In this study, we used continually passaged rat liver epithelial cells (WBs), which natively express the angiotensin type 1 receptor (AT1R) that served as a model cell system for studying 2ME2 mediated effects on AT1R expression. To validate the cell model, we performed additional studies in rat aortic smooth muscle cells to determine 2ME2’s effects on vascular cells. Our study demonstrated that 2ME2 treatment results in a 25–30% decrease in AT1R cell surface expression through a ERK1/2 dependent pathway. Furthermore, we observed similar results in primary rat aortic smooth muscle cells showing cardiovascular relevance. To our knowledge this is the first study of its kind observing the effects of 2ME2 on AT1R expression.
Materials and Methods
Materials
Continuously passaged rat liver epithelial cells (WB cells) were kindly provided by Dr. H. Shelton Earp, University of North Carolina at Chapel Hill (Chapel Hill, NC). Primary rat aortic smooth muscle cells were from Lonza (Walkersville, MD). Richter’s improved minimal essential medium was obtained from Cellgro-Mediatech Inc. (Manassas, VA). Fetal bovine serum (FBS) was from Equitech-Bio, Inc. (Kerrville, TX). Oligonucleotide primers and biotinated probes were obtained from Integrated DNA technologies, Inc. (Coralville, IA). Goat anti-rabbit Alexa-Fluor 488® IgG, 4′6′ diamidino 2-phenylindole-dihydrochloride (DAPI), and Prolong Gold Anti-Fade® were from Invitrogen (Carlsbad, CA). PCR master mix was from Roche (Indianapolis, IN). Losartan was provided by Merck Sharp & Dohme Research Laboratories (Rahway, NJ). Insulin, and gentamycin were from Sigma (St. Louis, MO). DNA/RNA extraction reagents were from Ambion/ABI (Austin, TX). [3H]AngII was from Amersham (Piscataway, NJ). Fura-2 AM, PD98059, and 2ME2 were from Calbiochem (La Jolla, CA). AT1R Antibody was from Santa Cruz Biotechnology (Santa Cruz, CA) and both anti-phospho-ERK1/2 and total ERK1/2 antibodies were from Cell Signaling (Danvers, MA). Electrophoresis regents were from Bio-Rad (Hercules, CA) and all other chemicals and molecular biology grade agents were purchased from Fisher Scientific (Fairlawn, NJ).
Methods
Cell culture
The continuously passaged rat liver epithelial cells (WB) were maintained in Richter’s Improved Minimum Essential Medium containing 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin at 37°C in 5% CO2 under 100% humidity. For primary culture of rat aortic smooth muscle cells, the cells were grown under similar conditions with the exception that the aortic smooth muscle cells were grown in 20% FBS. For the studies, cells were grown to 75–80% confluence, and 2-methoxyestradiol treatments were initiated in fresh medium, and grown for indicated times.
Radio-Labeled Ligand Binding Assay
Angiotensin II binding studies were performed in triplicate on WB cells grown in 6-well plates as described previously [41]. Briefly, cells grown to 70–80% confluency, then treated with 2ME2 for 24 hours. The cells were washed with phosphate buffered saline (PBS), and incubated at 22°C for 1 h with 0.05 nM [3H]AngII in binding buffer (50 mM Tris-HCl pH 7.4, 120 mM NaCl, 4 mM KCl, 1 mM CaCl2, 10 μg/ml bacitracin, 0.25% BSA and 2 mg/ml dextrose). In order to confirm specific binding, select control groups were incubated with unlabelled AngII (1 μM) for 15 min before the addition of radiolabeled AngII. Cells were washed 3 times with chilled PBS to remove nonspecifically bound AngII, and cells were lysed in 0.1% Triton-X-100 for 1 h. The lysate was collected transferred to counting vials, and radioactivity was determined using a Beckman® auto-gamma scintillation spectrometer. Specific [3H] binding was defined as that portion of the total binding displaced by 1 μM unlabelled AngII or 10 μM Losartan (AT1R specific antagonist). Competitive binding studies were performed as described above with logarithmic increases in unlabelled AngII, from 1 pM to 10 μM, in order to plot nonlinear regression curves from which we could calculate the dissociation constants (Kd) [42] and thus the relative affinities of AT1R among treated and untreated cells. Protein concentration was determined by using Bio-Rad® protein assay system based on the Bradford method [43].
Reverse Transcriptase (RT) Dual PCR
Cells were grown to 70–80% confluency and treated with or without 1 μM 2ME2 for 24 hours. Total RNA was isolated using guanidium thiocyanate-phenol-chlororom method described previously [44]. Total RNA was quantified and 5 μg/condition were processed for cDNA template conversion using MMLV-RT. The reaction without reverse transcriptase served as control for DNA contamination. The cDNA was then amplified with a dual-PCR primer set for AT1R and β-actin mRNA. AT1R primers (AT1R sense- 5′-TGATTCAGCTGGGCGTCATCCA-3′; AT1R antisense- 5′-TTTCGTAGACAGGC TTGAGTGGG-3′) were constructed based on the sequence of the rat vascular smooth muscle AT1AR cDNA [45]. Similarly, β-actin primers (β-actin sense – 5′-CGGAACCGCTCATTGCC-3′; β-actin antisense- 5′-ACCCACACTGTGCCCATCTA-3′) were constructed to amplify β-actin internal standard from mRNA coding sequence (accession#: AF122902). The PCR was performed in a 50 μl sample volume subjected to 30 cycles and the amplicons were analyzed on a 2% agarose ethidium bromide gel. Bands were visualized under UV to assure appropriate band density among β-actin controls. Intensity of bands were captured by the Bio-Rad Versa Doc® and quantified using Quantity One® software.
Measurement of Cytosolic Free Ca2+ Concentration
Cells were cultured in 6-well plates until 70–80% confluent and exposed to 2ME2 (1 μM) for 24 h in the presence or absence of PD98059 (20 μM). Cells were then rinsed with Hanks’ balanced salt solution (HBSS) and the assay was performed as described in Grynkiewicz et al. 1985. Briefly, cells were loaded with calcium dye Fura-2AM (1 μM) in Dulbecco’s modified phosphate buffered saline (DPBS with calcium) in a 37°C incubator with 5% CO2 and 100% humidity. After 1 h of incubation, cells were washed two times with DPBS. Additionally, cells were washed two times with assay buffer (145 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 10 mM glucose, 1.2 mM MgCl2, and 1.5 mM CaCl2.) and a final volume of 900 μl of assay buffer was added to each well. Baseline readings were taken at 340 nm excitation and 512 nm emission at 5 sec intervals using an ELISA plate reader (BioTek® SynergyMx). At the end of 2 min 100 μl of Ang II (10−6M) was added in each well and immediately readings were taken continuously at 5 sec intervals for an additional 5 min. At the end of the experiment, maximal emissions of calcium bound fura-2AM and free Fura 2AM were obtained by adding digitonin and EGTA to a final concentration of 0.22 and 1.15 mM, respectively. The intracellular concentration of calcium was determined using the formula, Δ[Ca2+]= ((Kd × (F − Fmin))/(Fmax − F)) − ((Kd × (Fx − Fmin))/(Fmax − Fx)) in which Kd = 224 nM, F corresponded to the fluorescent reading after addition of AngII, Fx corresponded to the fluorescent reading just before addition of AngII, Fmin the minimum reading after addition of EGTA and Fmax the maximum reading after the addition of digitonin.
Qualitative imaging of changes of intracellular Ca2+ by microscopy followed a similar procedure. WB cells grown to 70–80% confluence in 35 mm optical bottom plates (MatTek #P35G-0-10-C, Ashland, MA) and AngII induced changes in intracellular calcium were monitored using microspectrofluorometry method as described [46]. Cells were loaded with 1 μM fura2-AM in HBSS for 20 minutes. The cells were washed twice with HBSS and changes in intracellular Ca2+ were measured. The microscope’s emission wavelength was set at 510 nm and the excitation wavelengths at 340 and 380 nm. Excitation was monitored by a high-speed wavelength-switching device recorded with a CCD camera. Images were collected and analyzed using Slidebook® image analysis software.
Immunofluorescence Microscopy
WB cells were grown in chamber slides (Lab-Tek, Naperville, IL) to 75–80% confluency and exposed to 2ME2 (1 μM), PD 98059 (20 μM), or 2ME2 and PD98059 in combination. At the end of the 24 h cells were washed with ice-cold PBS, fixed with ethanol acetic acid mixture (3:1 by volume) or 3% paraformaldehyde in PBS for 30 min at 22°C. Cells were washed with chilled PBS (three times), blocked with 5% goat serum for 3 h, and incubated with primary antibody (dil. 1:100 anti-Total ERK1/2-Cell Signaling) overnight at 4°C. Cells were then washed 5 times with chilled PBS and incubated with Alexa Fluor® 488 fluorescent tagged secondary antibody (Invitrogen, Carlsbad, CA) for 1 h at 22°C dark followed by washing. In addition, nuclei were stained with 1nM 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and washed with ice-cold PBS. Slides were sealed with ProLong Gold® antifade mounting medium (Invitrogen, Carlsbad, CA) and visualized and photographed with a fluorescence microscope (Olympus IX70®) using a X20 objective equipped with and additional X1.5 magnification.
Phosphorylation of ERK1/2 as Determined by Western Blot Analysis
Cells were exposed to 2ME2 in the presence or absence of PD98059 (a MAP-kinase specific cell permeable inhibitor) for 24 hours and washed with phosphate- buffered saline. Cells were scraped in lysis buffer (10 mM TrisCl pH 7.4, 150 mM NaCl, 15% glycerol, 1% triton X-100, 1 mM sodium orthovanadate, 10 mg/ml leupeptin, 10 mg/ml aprotinin, 1 mM NaF, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Protein concentration was determined using Bio-Rad protein assay reagent based on the Bradford method [43]. Equal quantities of proteins were separated by 8% SDS-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and incubated with their respective primary antibodies. Immunoreactive bands were visualized using a chemiluminescence Western blotting system according to the manufacturer’s instructions (Amersham).
Data Analysis
Data were analyzed using GraphPad Prism® software. Results are presented as mean ± S.E.M and the value of P<0.05 was considered statistically significant. Analyses performed include parametric t-tests, first testing for Gaussian distribution, and one-way analysis of variance (ANOVA) with post-hoc Bonferroni analysis where appropriate according to previously established methods [47]. Values are normalized to milligrams of protein determined by Bio-Rad DC protein assay system based on the Bradford method [43]. For competitive binding studies, data were analyzed and non-linear regression curves were obtained as described previously [42].
Results
2ME2 Specifically Down-Regulates AT1R
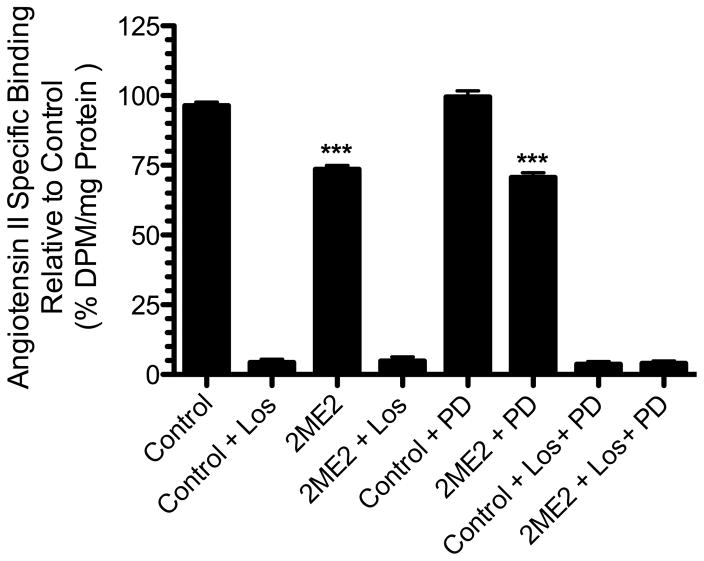
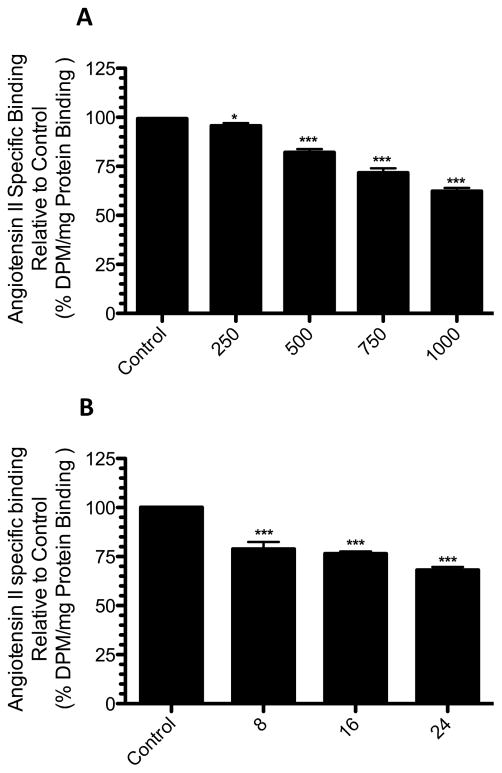
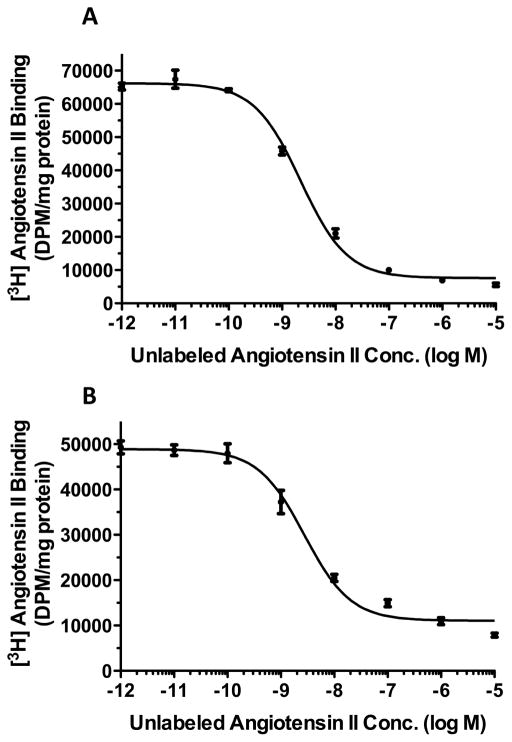
To determine the effects of 2ME2 on native AT1R expression, rat liver epithelial cells (WB) were exposed to 1μM 2ME2 for 24 hours and AngII specific binding was measured. The WB cell line was originally isolated by Dr. Grisham and coworkers in 1984 and is characterized as a normal male adult Fischer 344 rat liver epithelial diploid continuously passaged cell line [48] extensively used by Dr. Earp and associates, as well as other investigators, to delineate AngII mediated AT1R cell signaling and function [49–58]. In this study we used passaged cells to P-26, at which point there are no changes to the cell’s phenotype [52]. The results of these studies revealed a down-regulation of 22.79±1.789% (p<0.0001, n=9) in AngII specific binding with 2ME2 treatment alone. 10 μM Losartan, an AT1R antagonist, reduced AngII specific binding 92.09±1.672% (p<0.0001, n=9) and 91.55±1.944% (p<0.0001, n=9) in control and 2ME2 treated cells respectively. As >90% of the AngII binding is displaced by AT1R blockade, we concluded that radio-ligand binding in these cells is attributable primarily to AT1R binding. In order to assess any AT2R binding, we introduced 10 μM PD123319, an AT2R antagonist to both untreated and 2ME2 treated cells; we observed that there was no change brought about by AT2R antagonism in both control (mean difference between PD123319 and untreated control 3.094±2.542%, p=0.2412, n=9) and 2ME2 treated cells (mean difference between 2ME2+PD123319 and 2ME2 treated cells 2.985±2.136%, p=0.1814, n=9). It was therefore concluded that radio-ligand binding was significantly affected by 2ME2, but as AT2R expression or binding was not significant in this cell type, any effect on binding by 2ME2 was specific to AT1R [Fig 1]. In the next experiment, we determined how this AT1R down-regulation was related to both 2ME2 concentration and treatment time. Cells were exposed to 2ME2 at indicated concentrations for 24 h [Fig 2A] or exposed to 1 μM 2ME2 for indicated times [Fig 2B], after which radio-ligand binding measurements were made. The results of radio-ligand binding with increasing concentrations at an interval of 250 nM demonstrated that a significant down-regulation occurred at all concentrations used (250 nM binding reduced 3.611±1.379%, p=0.0187; 500 nM binding reduced 17.18±1.709%, p<0.0001; 750 nM binding reduced 27.60±2.325%, p<0.0001; 1000 nM binding reduced 36.92±1.670%, p<0.0001, n=9). For further analyses, we chose a concentration of 1000 nM or 1 uM. The results of the time-course study demonstrated that at 8 h intervals, 1 uM 2ME2 significantly down-regulated AT1R expression (8 h treatment binding reduced 21.26±3.614%, p<0.0001; 16 h treatment binding reduced 23.60±1.145%, p<0.0001; 24 h treatment binding reduced 31.91±1.539%, p<0.0001, n=8). Moreover, the progressive decrease in AT1R binding over time suggests that 2ME2 induced down-regulation of AT1R surface expression is time, as well as dose-dependent. Thus far the previous studies indicated that significantly less AngII was capable of binding to the surface of the WB cells upon treatment with 2ME2; however, this finding was not necessarily due to decreased surface expression of the receptor, but rather an altered affinity of the AT1R for its ligand due to differential coupling of the receptor with G-proteins. We conducted competitive radio-ligand binding studies in order to elucidate the relative affinities of AT1R in 2ME2 treated and untreated cells [Fig 3]. The results of these analyses demonstrated that the Kd of untreated control cells’ AT1R was within normal affinity for AngII at 3.321±0.5545 nM, as was the calculated Kd in the 1 μM 2ME2 treated cells after 24 h at 3.568±0.6955 nM. Comparing the results from 3 individual experiments revealed that there was no significant difference between the calculated Kd in control cells from that of 2ME2 treated cells (mean difference of 7.98±20.94%, p=0.7225, n=3). Therefore, the results of this final radio-ligand binding study demonstrated that the affinity is not significantly affected upon 2ME2 treatment.
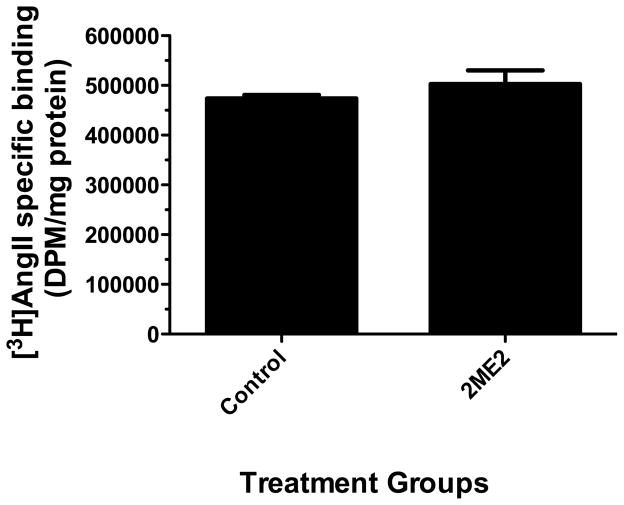
Figure 1. 2ME2 inhibits AT1R specific binding in WB cells.
Radio-ligand binding assay after 24 h 1 μM 2-methoxyestradiol (2ME2) treatment and losartan (Los), or PD123319 (PD) blockade. Cells were exposed to 2ME2 (1 μM) for 24 h and [3H]AngII binding measured in the presence or absence of losartan (an AT1R specific antagonist) or PD123319 (an AT2R specific antagonist). (n=9). Data are expressed as mean ± SEM ***p<0.0001 versus untreated control.
Figure 2. 2ME2 results in AT1R down-regulation in a dose and time dependent manner.
(A) 2ME2 mediated AT1R inhibitory effect is dose dependent. Radio-ligand binding assay after variable concentrations (250 – 1000 nM) of 2ME2 treatment for 24 h. (n=9). (B) 2ME2 mediated AT1R inhibitory effect is time dependent. Radio-ligand binding assay after exposure to 1 μM 2ME2 for variable treatment times as indicated. (n=8) Data are expressed as mean ± SEM ***p<0.0001 versus untreated control.
Figure 3. Competition binding studies reveal no change in receptor affinity after 2ME2 treatment.
Radio-ligand binding competition studies performed on untreated cells (A) and cells treated with 1 μM 2ME2 for 24 hours (B). Nonlinear least squares regression analysis gave a Kd of 3.321±0.5545 nM for receptors in cells exposed to normal medium and a Kd of 3.568±0.6955 nM for receptors in cells exposed to 2ME2 (n=3).
2ME2 Significantly Inhibits AngII-Mediated Increase in Intracellular Ca2+
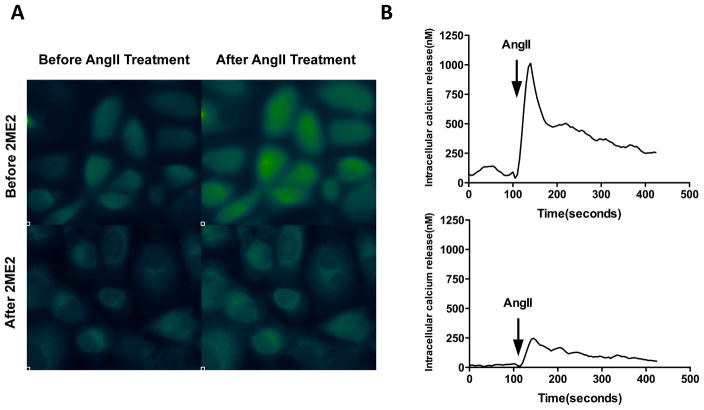
Through the previous analyses, we demonstrated that 2ME2 is capable of significantly reducing the cell-surface expression of AT1R in WB cells. However, the significance of this reduction was dependent upon a concomitant reduction in the cell’s responsiveness to AngII stimulation. For this purpose, we performed a fluorometric Ca2+ efflux study in order to ascertain the relative responsiveness to applied AngII treatment. Qualitative analysis of Ca2+ fluorometric microscopy demonstrated that there was an observable decrease in the Ca2+ released into the cytoplasm upon stimulation of AT1R by AngII [Fig. 4A]. This result supported our previous finding that 2ME2 treatment results in a decrease in the expression of AT1R on the cell surface. In order to validate this finding, we performed quantitative analysis using a similar technique in conjunction with an ELISA plate reader. The results demonstrate that a 78.65±16.11% (p=0.0081, n=3) reduction in Ca2+ release takes place when cells are treated with 2ME2 [Fig. 4B]. The resultant decrease in Ca2+ release correlated with the previous observation of reduced surface expression elicited by 2ME2 treatment.
Figure 4. Down-regulation of AT1R by 2ME2 produces an associated decrease in AngII elicited G-protein coupled response.
(A) Fluorescent imaging shows 1 μM 2ME2 exposure for 24 h significantly inhibited AngII mediated increase in intracellular Ca2+, a G-protein coupled response. Transient free Ca2+ was measured with the Ca2+ indicator fura 2-AM using fluorescent imaging microscopy before and after AngII stimulation (10−7 M), with or without 2ME2 treatment. Ca2+ bound fura 2-AM emits green fluorescence. (B) Ca2+ FLIPR assay shows a significant reduction in fluorescent emission after 1 μM 2ME2 exposure for 24 h. Representative tracing of AngII mediated transient increase in intracellular Ca2+ without (upper) and with (lower) 2ME2 treatment. The tracings are representative of 3 separate experiments; an overall reduction of 78.65±16.11%.
2ME2-Elicited Down-Regulation Is Dependent on the Native Rat Promoter
In order to elucidate the nature of 2ME2 induced AT1R down-regulation, we performed an experiment in which Chinese hampster ovarian (CHO) cells expressing recombinant AT1R protein (T24CHO/AT1A) linked to a constitutively active cytomegalovirus (CMV) promoter were exposed to 1 μM 2ME2. The cells were then assayed for radio-ligand binding and receptor expression relative to protein. The results revealed that these cells were 2ME2 insensitive at an equivalent dose and time (mean difference 5.043±12.94%, p=0.7018, n=9) [Fig. 5]. From this result, we concluded that 2ME2 is exerting its effect directly on the promoter of the rat AT1R and suggested that down-regulation of AT1R by 2ME2 is through regulation of AT1R mRNA transcription.
Figure 5. 2ME2 treatment does not result in AT1R down-regulation in T3CHO/AT1A linked to CMV promoter.
Radio-ligand binding assay performed after 24 h 1 μM 2ME2 treatment. Mean difference between treated and untreated control 5.043±12.94%, p=0.7018 (n=9).
2ME2 Down-Regulates AT1R in an ERK1/2 Dependent Manner
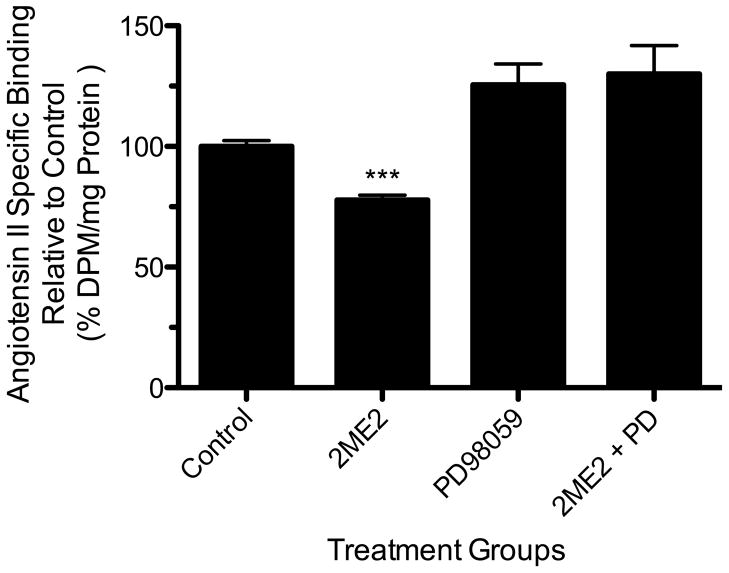
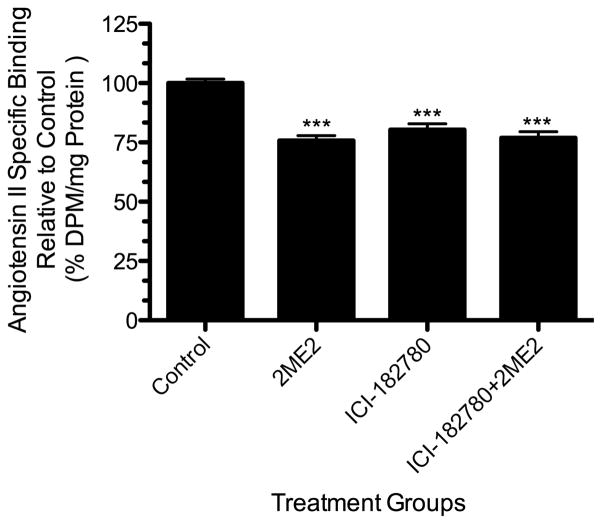
To determine the 2ME2-associated cellular signaling responsible for AT1R mRNA and protein down-regulation, we focused on extracellular signal-regulated kinases 1 and 2 (ERK1/2, also referred to as MAP Kinase p42/p44), which was shown previously to play a role in glucose induced AT1R down-regulation [57]. Cells were grown to 70–80% confluence in complete media and exposed to 2ME2. 24-hour treatment with 1 μM 2ME2 induced ERK1/2 phosphorylation without any change in the total protein [Fig. 6A]. This 2ME2-induced phosphorylation was completely inhibited by MEK inhibitor PD98059 (20 μM). To further confirm 2ME2-induced activation of ERK1/2, we utilized immunofluorescent microscopy and monitored translocation of ERK1/2 after 2ME2 application. The result shows that 2ME2 not only activates ERK1/2, but induces its translocation to the nucleus [Fig. 6B]. This effect is reversible if the cells are pretreated with 20 μM PD98059. We performed mRNA analysis in order to determine whether 2ME2 affects the transcription of AT1R mRNA and consequently the rate of protein production, and if so, whether this effect may be reversed by MEK inhibition. We carried out (RT) dual-PCR analysis on AT1R mRNA with and without 2ME2 treatment parallel to PD98059 treated groups. The housekeeping gene β-actin mRNA is not affected by 2ME2 and is therefore used as an mRNA quantitation standard. (RT) dual-PCR was performed in a single reaction and analyzed by ethidium bromide 2.0% agarose gel electrophoresis. Densitometric analysis of multiple experiments (n=3) revealed that 2ME2 exposure resulted in a 40.99±6.756% (p<0.0037) reduction of AT1R mRNA transcript compared to control [Fig. 7]. Upon MEK inhibition, 2ME2 treated cells’ AT1R mRNA levels were not significantly different than those in cells in which MEK inhibition was not augmented by 2ME2 treatment (mean difference 5.640±15.65% between PD and PD+2ME2 treated cells, p=0.7368, n=3). Quantitative calcium analysis revealed that there was significant reversal of the 2ME2-mediated reduction in Ca2+ signaling (mean difference 2.209±29.10% between PD98059 and PD98059+2ME2 treated cells, p=0.9431, n=3) [Fig 8]. Under similar conditions, radio labeled binding data showed that there was little to no effect by 2ME2 on binding of radio-labeled AngII when MEK signaling was inhibited in the presence of 20 μM PD98059 (mean difference between PD98059 treated and PD98059+2ME2 treated cells 4.424±14.60%, p=0.7658, n=9) [Fig. 9]. From these analyses we concluded that ERK1/2 is activated upon exposure to 2ME2 and translocates to the nucleus. Upon translocation, ERK1/2 then directly or indirectly affects the transcription and thus the expression of the AT1R protein, as 2ME2 induced down-regulation is eliminated upon blockade of MAP Kinase Kinase, (MEK) and subsequently ERK1/2. To further investigate the mechanism of 2ME2 induced down-regulation of AT1R, we performed additional binding studies using the ERα/ERβ antagonist ICI182780 (50 μM) [Fig. 10]. The results demonstrate that ER blockade has no restorative effect on [3H]AngII binding (reduction of binding from control by 2ME2 treatment, 24.2±2.633% [p<0.001, n=3], and after ER blockade, 23.02±3.062% [p<0.001, n=3]). Interestingly, ICI182780 treatment alone resulted in a significant reduction in [3H]AngII binding (mean reduction in binding from control after ICI treatment, 19.64±3.012% [p<0.001, n=3]).
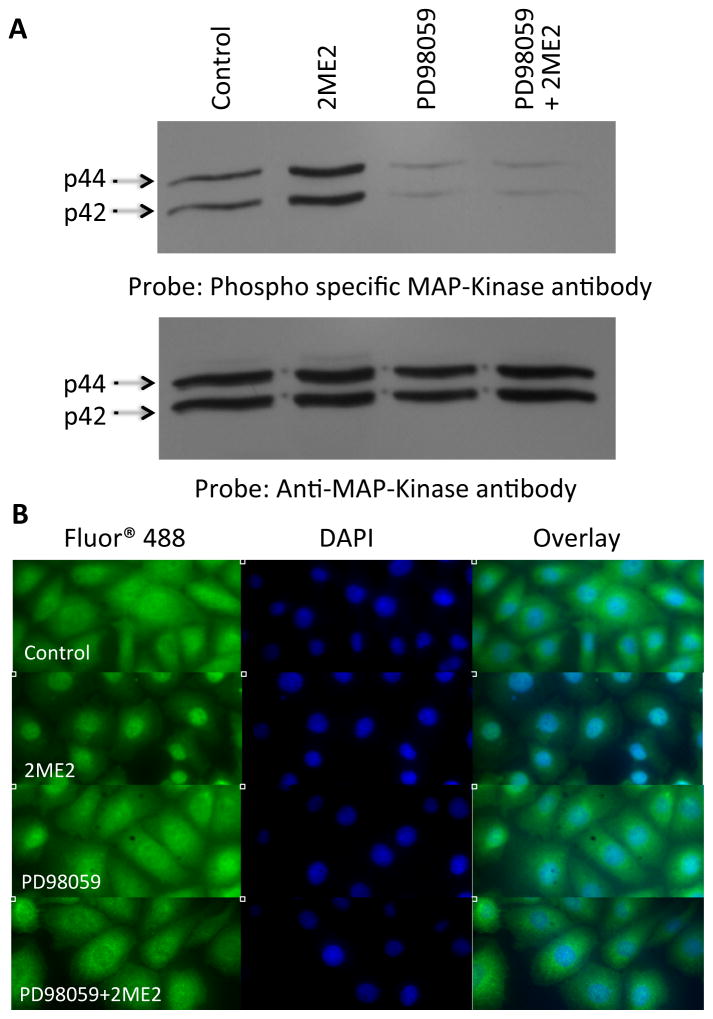
Figure 6. 2ME2 induces phosphorylation of ERK1/2.
(A) Total cell lysates were prepared from control, 2ME2 treated, MEK inhibitor PD98059 treated, and 2ME2+PD98059 treated cells and immunoblotted with phospho-specific ERK1/2 antibody (upper). Blot stripped and reprobed with anti-total ERK1/2 antibody to demonstrate equal loading (lower). A representative blot is shown (n=3). (B) ERK1/2 translocates to the nucleus upon treatment with 1 μM 2ME2. Immunofluorescent staining using primary rabbit anti-ERK1/2 IgG followed by secondary anti-rabbit IgG conjugated with Alexa Fluor 488®. Nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI). Treatment groups included are untreated control, 2ME2 treatment, PD98059 treatment, and combined 2ME2 and PD98059 treatment. Images are divided among FITC, DAPI, and overlayed images to demonstrate nuclear co-localization. Representative images are shown of 3 individual experiments.
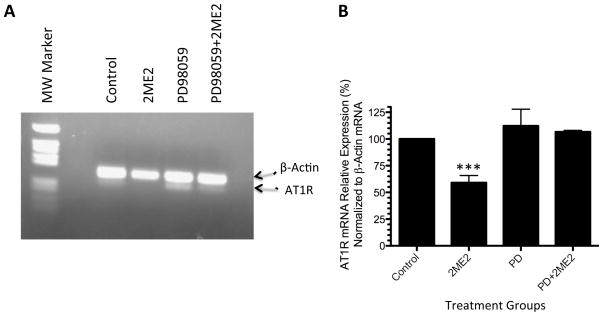
Figure 7. 2ME2 inhibits AT1R mRNA expression.
(A) Representative image of ethidium bromide gel of a dual RT-PCR reaction using AT1R/actin specific primers to determine the effects of 2ME2 on AT1R mRNA in WB cells. Bands detected are at 289 and 206 bp for β-actin and AT1R mRNAs respectively. (B) Quantitation of multiple analyses of AT1R expression were normalized to β-actin and data are expressed as mean ± SEM, (n=3). Densitometric analysis of multiple experiments (N=3) revealed that TA exposure resulted in a 40.99±6.756% (p=0.0037). **p<0.001 compared to untreated control.
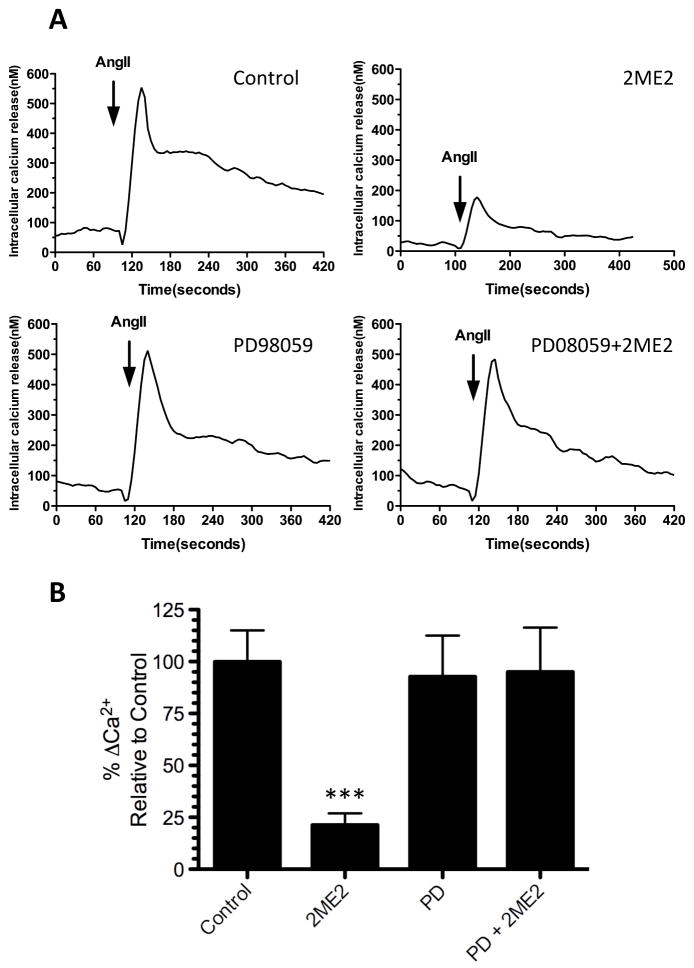
Figure 8. Ca2+ FLIPR assay shows MEK inhibitor PD98059 restores intracellular Ca2+ release in 2ME2 treated cells.
Increase in intracellular Ca2+ was measured as described in the methods section. (A) Representative tracings of transient increase in intracellular Ca2+ (upper left) in untreated control cells, (upper right) 2ME2 treated cells, (lower left) PD98059 treated cells, and (lower right) PD98059+2ME2 treated cells. (B) Compiled data of 3 experiments and expressed at relative AngII elicited Ca2+ release as compared to untreated control. Mean difference between PD98509+2ME2 and PD treated control 2.209±29.10%, p=0.9431. Data are expressed as mean ± SEM. **p<0.001 compared to control.
Figure 9. MEK inhibition restores radio-ligand binding in 2ME2 treated cells.
Radio-ligand binding assay was performed after 24 h 1 μM 2ME2 treated and untreated cells in the presence or absence of PD98059 (20 μM). Mean difference between PD98059+2ME2 and PD98059 control is 4.424±14.60%, p=0.7658 (n=9). Data are expressed as mean ± SEM.
Figure 10. ERα/ERβ antagonism does not restore radio-ligand binding in 2ME2 treated cells.
Radio-ligand binding assay was performed after 24 h 1 μM 2ME2 treated and untreated cells in the presence or absence of ICI182780 (50 μM). Mean difference between ICI182780+2ME2 and untreated control is 23.02±3.062%, p<0.001 (n=9). Data are expressed as mean ±SEM.
2ME2 Induced Effects On Primary Vascular Smooth Muscle Cells
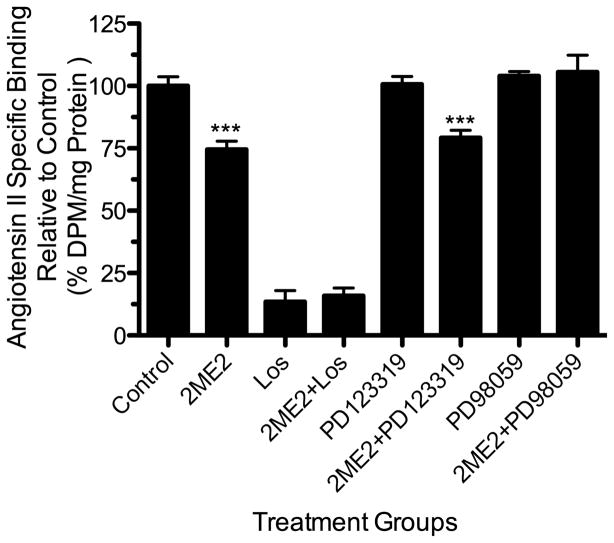
To validate the results obtained in the WB cell model, and thus create relevance to vasculature, we performed additional studies using primary rat aortic smooth muscle cells (RASMC) [Fig. 11]. The results show that 2ME2 is capable of down-regulating AT1R in primary rat vascular smooth muscle cells. The study validated our previous findings that the down-regulation by 2ME2 is AT1R specific and is mediated by ERK1/2 activation. Upon treatment with 1 μM 2ME2, AngII binding in RASMC decreased by 24.20±5.789% (p=0.0019, n=3). AT2R blockade by PD123319 had no effect in control cells (mean difference in control and PD123319 treated cells 5.600±5.657%, p=0.3455, n=3), whereas losartan blockade significantly reduced total binding (mean difference in control and losartan treated cells 95.20±5.370%, p<0.0001, n=3). Losartan treatment caused no significant difference in 2ME2 treated cells compared to control losartan treated cells (p=0.2330, n=3). Finally, MEK inhibition and subsequent ERK1/2 inhibition resulted in restoration of binding in 2ME2 treated cells when compared to control with no significant difference found between the two groups (p=0.8554, n=3).
Figure 11. 2ME2 treatment down-regulates radio-ligand binding in rat aortic smooth muscle cells.
Cells were exposed to 2ME2 (1 μM) for 24 h and [3H]AngII binding measured in the presence or absence of losartan (an AT1R specific antagonist) PD123319 (an AT2R specific antagonist), or PD98059 (a specific MEK inhibitor). (n=9). Data are expressed as mean ± SEM ***p<0.0001 versus untreated control.
Discussion
The primary objective of the present study was to determine the effects, if any, of 2ME2 on AT1R expression. We used continuously passaged rat liver epithelial cells (WB), which naturally express AT1R, as evident by the fact that more than 90% of radio-labeled AngII binding is AT1R specific antagonist losartan sensitive. 2ME2 treatment for 24 hours resulted in a significant reduction of AT1R expression in a dose and time dependent manner. Consistently with the reduction of receptor density on the cell surface, we observed a significant reduction in AT1R mRNA in 2ME2 treated cells; however, when cells expressing recombinant AT1R linked with a constitutively active CMV promoter, 2ME2 did not down-regulate AT1R expression. From these results, we concluded that 2ME2 down-regulates AT1R expression by inhibition of AT1R gene transcription. In addition, we observed that 2ME2 resulted in a significant activation of ERK1/2, and from studies in which this MAP Kinase is selectively inhibited, we show that this activation is essential for the signaling pathways eventually leading to the down-regulation of AT1R gene transcription. Furthermore, our study shows this effect is ERα/ERβ independent and reproducible in primary rat aortic smooth muscle cells of cardiovascular relevance. The present study’s findings may have substantial importance in understanding estrogen’s beneficial cardiovascular effects, as well as providing a potential therapeutic agent targeting gene expression, therefore introducing a new paradigm into the current pharmacopoeia targeting the renin-angiotensin system.
Agents that disrupt AT1R stimulation have profound protective effects in pathologically affected tissues relative to AngII signaling [60]. With the discovery of membrane associated estrogen receptors [61] and our findings here presented, 2ME2 has potential applications for the treatment of hypertension, or in fact any condition either caused or exacerbated by AngII stimulation of AT1R. Moreover, as this study is restricted to a single cell type, the findings may be limited to liver tissue. However, if these findings are significant to liver tissue only, which is unlikely, this does not necessarily mean that the results of this study are without clinical significance. Studies have shown that following AT1R blockade, hepatocytes preconditioned to develop liver fibrosis and steatosis have significant attenuation of these inflammatory conditions [62], showing at once that chronic inflammatory liver diseases are exacerbated by AngII stimulation of the AT1R and that the progression of these conditions is significantly altered by inhibition or interruption of AT1R signaling.
As for the approved uses of 2ME2, clinical trials have been conducted, though interestingly, not for any known effect on the expression of AngII receptors or cardiovascular disorders [63,64]. 2ME2 has few or no feminizing effects [65], and therefore it may have equally beneficial effects in males and females alike; however, further clinical trials should reveal the feasibility for pan-gender acceptability. 2ME2 has been shown to be a promising anti-cancer agent [66] and is available by brand name Panzem (EntreMed, Inc.) for the treatment of ovarian cancer as an orphan drug indication approved by the FDA, but its role in the treatment of hypertension is yet unknown and unsuspected. This is not surprising as 2ME2’s mechanism of action and (possible) diverse effects are unknown. Interestingly, 2ME2 and estradiol have shown acute vasodilatory effects on rat aortic smooth muscle [67]. We may reiterate that these effects are classified as acute as the study mentioned determined that 2ME2 incubation significantly attenuated vasoconstriction elicited by stimulation with phenylephrine. From analysis of the data from this study, the authors concluded that significant eNOS activation and subsequent nitric oxide (NO) resulted in the vasodilation. However, our own findings are distinct as determined by the time-course followed by the 2ME2 mediated down-regulation of AT1R, a process taking hours to complete (from 8 to 24 h). An important note we may interject is that vascular tone differs significantly based upon gender, suggesting that female endocrinology contributes significantly to greater vasodilation and relaxation [68]. Studies in the past have shown that upon castration, no significant difference was detected between male subjects’ vascular tone but upon ovariectomization, females showed significantly enhanced vascular tone [69,70]. The ultimate conclusion from these studies was that differences in vascular tone in these test groups, from gonad intact to gonadectomized females, was not due to androgen signaling but rather to the enigmatic vasodilatory effect of estrogen. It may be that estrogen and 2ME2 have NO-related acute effects as well as long-term alterations in AT1R distribution, though resolution of these diverse mechanisms remains to be determined. However, we may use a study correlating 2ME2 with a pathophysiology known to be associated with AT1R expression. Pre-eclampsia is characterized by hypertension and proteinuria and is associated with trophoblast invasion and improper spinal arterial remodeling [71–73]. Researchers have demonstrated that injection of Th1 lymphocytes in order to elicit conditions mimicking the conditions of pre-eclampsia significantly up-regulated AT1R expression in the kidney and placenta [74]. The hypertensive effects have been specifically linked to the renin-angiotensin system, wherein there is an imbalance of vasoconstrictors and vasoactivators, ultimately leading to AngII overwhelming the inherent relaxation mediated by induction of eNOS [75,76] Additionally, catechol o-methyltransferase (COMT) deficient mice are significantly predisposed to pre-eclampsia [77]. Moreover, when these mice are supplemented with 2ME2, the characteristic rise in blood pressure in COMT−/− mice does not occur, establishing that not only 2ME2 restored these knockout mice to control levels, but also made an interesting proposal that 2ME2 may be a physiologically significant metabolite at naturally occurring (i.e. non-pharmacological) titers. However, this study focused on the effect of 2ME2 at pharmacological concentrations with the intention of elucidating the therapeutic potential of synthetic 2ME2 supplementation, and studies similar to our own evaluating 2ME2’s effects on other targets repeatedly use concentrations within the range presented in this study [36,37,39,40]. Validation of these in vitro findings in vivo are as yet unperformed.
With regard to cellular signaling, previous literature suggests that methoxyestradiols inhibit the proliferation and hypertrophy of vascular smooth muscle cells and cardiac fibroblasts, inevitably affecting the physiology of these tissues and the heart and kidney in particular [78]. 2ME2 has been shown previously to increase the activity of ERK1/2 in SW-13 adrenal carcinoma cells [26], but as to how 2ME2 activates these MAP Kinases, much remains unclear. Interestingly, recent studies have proposed that estrogens may act, in addition to the binding and activation of nuclear receptors, through a novel 7-transmembrane G-protein coupled receptor, GPR30 [79], and further investigation of this new receptor may elucidate the elusive mechanism of action of 2ME2. GPR30-dependent activation of ERK1/2 has been described in a previous study through the transactivation of EGFR [80], which was also associated with another signaling mechanism by EGFR-mediated activation of phosphatidylinositol-3 kinase (PI3K) activation [81]. Based on our study using the ERα/ERβ antagonist ICI182780, a known GPR30 agonist [82], it is conceivable that 2ME2’s mechanism in the observed studies involves activation of GPR30 as ICI182780 treatment resulted in a similar down-regulation of AT1R binding, independent of 2ME2. Further studies are needed to confirm this observation. The actual classification of GPR30 remains unknown, but a pair of studies support that GPR30 activation initiates adenylate cyclase activation, thereby increasing the supply of cytosolic cAMP [82,83]. A study previously found that estradiol-mediated inhibition of smooth muscle cell growth was attributable in part by an increase in cAMP [84]; therefore, the initial observation in this study correlates with the ER-independent mediated effects by estradiol and its metabolites. From the findings in the present study and the tantalizing clues from previous studies and the simultaneous dearth of information of cellular signaling elicited by 2ME2, we may conclude that further studies may reveal very interesting results as that may resolve important issues in the realm of the renin-angiotensin system, female endocrinology, and ultimate benefits provided via hormone replacement therapy. In conclusion, our results indicate that 2ME2 is capable of inducing AT1R down-regulation. As AT1R plays a central role in the development of and progression of cardiovascular and inflammatory diseases, 2ME2-elicited changes in receptor expression may supplement current approaches in the panoply of hormone replacement therapies, maximizing their protective cardiovascular effects while minimizing the risks. However, further studies are needed to validate these findings as well as to determine the role of 2ME2 receptors and their associated signaling pathways in transcriptional down-regulation of AT1R.
Acknowledgments
This study was supported in part by a grant from the National Institute of Health (DK072140) and a graduate fellowship from the Texas Tech University School of Pharmacy to S.K. and R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams GH, Dluhy RG. Disorders of the Adrenal Cortex. 17. New York: McGraw Hill Companies Inc; 2008. [Google Scholar]
- 2.Powers AC. Diabetes Mellitus. 17. New York: McGraw Hill companies Inc; 2008. [Google Scholar]
- 3.Wang CH, Li F, Takahashi N. The renin angiotensin system and the metabolic syndrome. Open Hypertens J. 2010;3:1–13. doi: 10.2174/1876526203010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura K, Tanaka Y, Tsurumi Y, et al. The role of angiotensin AT1 receptor-associated protein in renin-angiotensin system regulation and function. Curr Hypertens Rep. 2007;9:121–7. doi: 10.1007/s11906-007-0022-6. [DOI] [PubMed] [Google Scholar]
- 5.Henrion D, Kubis N, Lévy BI. Physiological and pathophysiological functions of the AT(2) subtype receptor of angiotensin II: from large arteries to the microcirculation. Hypertension. 2001;38(5):1150–7. doi: 10.1161/hy1101.096109. [DOI] [PubMed] [Google Scholar]
- 6.Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res. 2009;81(3):592–600. doi: 10.1093/cvr/cvn230. [DOI] [PubMed] [Google Scholar]
- 7.Siragy HM. Evidence for benefits of angiotensin receptor blockade beyond blood pressure control. Curr Hypertens Rep. 2008;10(4):261–7. doi: 10.1007/s11906-008-0050-x. [DOI] [PubMed] [Google Scholar]
- 8.Marni F, Wang Y, Morishima M, Shimaoka T, Uchino T, Zheng M, Kaku T, Ono K. 17 beta-estradiol modulates expression of low-voltage-activated Ca(V)3.2 T-type calcium channel via extracellularly regulated kinase pathway in cardiomyocytes. Endocrinology. 2009;150(2):879–88. doi: 10.1210/en.2008-0645. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 10.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297(5):H1806–13. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson SH, Bandera EV, Orlow I. Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am J Epidemiol. 2007;165(3):235–45. doi: 10.1093/aje/kwk015. [DOI] [PubMed] [Google Scholar]
- 12.Zhu BT, Conney AH. Is 2-Methoxy Estradiol and endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Research. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 13.Berg D, Thaler F, Kuss E. Concentrations of 2-hydroxyoestrogens in human sera measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh) 1982;100(1):154–60. doi: 10.1530/acta.0.1000154. [DOI] [PubMed] [Google Scholar]
- 14.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–90. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 15.Bjornstrom L, Sjoberg M. Mechansims of Estrogen receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Molecular Endocrinology. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 16.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 17.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- 18.Teyssier C, Belguise K, Galtier F, Chalbos D. Characterization of the physical interaction between estrogen receptor and JUN proteins. J Biol Chem. 2001;276:36361–36369. doi: 10.1074/jbc.M101806200. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 21.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111(11):1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawagoe J, Ohmichi M, Takahashi T, Ohshima C, Mabuchi S, Takahashi K, Igarashi H, Mori-Abe A, Saitoh M, Du B, Ohta T, Kimura A, Kyo S, Inoue M, Kurachi H. Raloxifene inhibits estrogen-induced up-regulation of telomerase activity in a human breast cancer cell line. J Biol Chem. 2003;278(44):43363–72. doi: 10.1074/jbc.M304363200. [DOI] [PubMed] [Google Scholar]
- 23.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 25.Neary JT. MAPK cascades in cell growth and death. News in Physiol Sci. 1997;12:286–293. [Google Scholar]
- 26.Brown JW, Kesler CT, Neary JT, Fishman LM. Effects of Androgens and Estrogens and Catechol and Methoxy-Estrogen Derivatives on Mitogen Activated Protein Kinase (ERK 1,2) Activity in SW-13 in Human Adrenal Carcinoma Cells. Horm Metab Res. 2001;33(3):127–30. doi: 10.1055/s-2001-14937. [DOI] [PubMed] [Google Scholar]
- 27.Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C, calcium pathway. J Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325(11):756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 29.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273(3):199–208. [PubMed] [Google Scholar]
- 30.Kolovou G, Giannakopoulou V, Vasiliadis Y, Bilianou H. Effects of estrogens on atherogenesis. Curr Vasc Pharmacol. 2011;9(2):244–57. doi: 10.2174/157016111794519327. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan D, Catley L, Hideshima T, Li G, Leblanc R, Gupta D, Sattier M, Richardson P, Schlossman RL, Podar K, Weller E, Munshi N, Anderson KC. 2-methoxyestradiol overcomes drug resistance in multiple myeloma. Blood. 2002;100:2187–2194. doi: 10.1182/blood-2002-02-0376. [DOI] [PubMed] [Google Scholar]
- 32.Dingli D, Timm M, Russell SJ, Witzig TE, Rajkumar SV. Promising preclinical activity of 2-methoxyestradiol in multiple myeloma. Clin Cancer Res. 2002;8:3948–3954. [PubMed] [Google Scholar]
- 33.Stubelius A, Andréasson E, Karlsson A, Ohlsson C, Tivesten A, Islander U, Carlsten H. Role of 2-methoxyestradiol as inhibitor of arthritis and osteoporosis in a model of postmenopausal rheumatoid arthritis. Clin Immunol. 2011;140(1):37–46. doi: 10.1016/j.clim.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15(1):76–83. doi: 10.1159/000135627. [DOI] [PubMed] [Google Scholar]
- 35.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–72. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 36.Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37(2 Part 2):640–4. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- 37.Dubey RK, Gillespie DG, Jackson EK, Keller PJ. 17Beta-estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension. 1998;31(1 Pt 2):522–8. doi: 10.1161/01.hyp.31.1.522. [DOI] [PubMed] [Google Scholar]
- 38.Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen-activated protein kinase activity. Arterioscler Thromb Vasc Biol. 2000;20(4):964–72. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- 39.Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Korzekwa KR, Fingerle J, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of estradiol on vascular smooth muscle cells via estrogen receptor-independent mechanisms. Biochem Biophys Res Commun. 2000;278(1):27–33. doi: 10.1006/bbrc.2000.3755. [DOI] [PubMed] [Google Scholar]
- 40.Barchiesi F, Lucchinetti E, Zaugg M, Ogunshola OO, Wright M, Meyer M, Rosselli M, Schaufelberger S, Gillespie DG, Jackson EK, Dubey RK. Candidate genes and mechanisms for 2-methoxyestradiol-mediated vasoprotection. Hypertension. 2010;56(5):964–72. doi: 10.1161/HYPERTENSIONAHA.110.152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thekkumkara TJ, Cookson R, Linas SL. Angiotensin (AT1A) receptor-mediated increases in transcellular sodium transport in proximal tubule cells. Am J Physiol. 1998;274(5 Pt 2):F897–905. doi: 10.1152/ajprenal.1998.274.5.F897. [DOI] [PubMed] [Google Scholar]
- 42.Swillens S. How to estimate the total receptor concentration when the specific radioactivity of the ligand is unknown. Trends Pharmacol Sci. 1992;13(12):430–4. doi: 10.1016/0165-6147(92)90139-w. [DOI] [PubMed] [Google Scholar]
- 43.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 44.Thekkumkara TJ, Linas SL. Evidence for involvement of 3′-untranslated region in determining angiotensin II receptor coupling specificity to G-protein. Biochem J. 2003;370(Pt 2):631–9. doi: 10.1042/BJ20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351(6323):233–6. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 46.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–50. [PubMed] [Google Scholar]
- 47.Snyder R, Thekkumkara TJ. 13-Cis Retinoic Acid Specific Down-Regulation of Angiotensin Type 1 Receptor in Rat Liver Epithelial and Aortic Smooth Muscle Cells. J Mol Endocrinol. 2011 doi: 10.1530/JME-11-0095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res. 1984;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Lee JW, Graves LM, Earp HS. Angiotensin II stimulates ERK via two pathways in epithelial cells: protein kinase C suppresses a G–protein coupled receptor EGF–receptor transactivation pathway. EMBO Journal. 1998;17:2574–2583. doi: 10.1093/emboj/17.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earp HS, Huckle WR, Dawson TL, Li X, Graves LM, Dy R. Angiotensin II activates at least two tyrosine kinases in rat liver epithelial cells. Separation of the major calcium-regulated tyrosine kinase from p125FAK. J Biol Chem. 1995;270(47):28440–7. doi: 10.1074/jbc.270.47.28440. [DOI] [PubMed] [Google Scholar]
- 51.Huckle WR, Dy RC, Earp HS. Calcium-dependent increase in tyrosine kinase activity stimulated by angiotensin II. Proc Natl Acad Sci USA. 1992;89(18):8837–41. doi: 10.1073/pnas.89.18.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huckle WR, Prokop CA, Dy RC, Herman B, Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990;10(12):6290–8. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Earp HS. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272(22):14341–8. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Yu H, Graves LM, Earp HS. Protein kinase C and protein kinase A inhibit calcium-dependent but not stress-dependent c-Jun N-terminal kinase activation in rat liver epithelial cells. J Biol Chem. 1997;272(23):14996–5002. doi: 10.1074/jbc.272.23.14996. [DOI] [PubMed] [Google Scholar]
- 55.McCune BK, Earp HS. The epidermal growth factor receptor tyrosine kinase in liver epithelial cells. The effect of ligand-dependent changes in cellular location. J Biol Chem. 1989;264(26):15501–7. [PubMed] [Google Scholar]
- 56.Tsao MS, Earp HS, Grisham JW. The effects of epidermal growth factor and the state of confluence on enzymatic activities of cultured rat liver epithelial cells. J Cell Physiol. 1986;126(2):167–73. doi: 10.1002/jcp.1041260204. [DOI] [PubMed] [Google Scholar]
- 57.Bokkala S, Joseph SK. Angiotensin II-induced down-regulation of inositol trisphosphate receptors in WB rat liver epithelial cells. Evidence for involvement of the proteasome pathway. J Biol Chem. 1997;272(19):12454–61. doi: 10.1074/jbc.272.19.12454. [DOI] [PubMed] [Google Scholar]
- 58.Bokkala S, Reis HM, Rubin E, Joseph SK. Effect of angiotensin II and ethanol on the expression of connexin 43 in WB rat liver epithelial cells. Biochem J. 2001;357(Pt 3):769–77. doi: 10.1042/0264-6021:3570769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SH, Woo CH, Kim JH, Lee JH, Yang IS, Park KM, Han HJ. High glucose down-regulates angiotensin II binding via the PKC-MAPK-cPLA2 signal cascade in renal proximal tubule cells. Kidney Int. 2002;61(3):913–25. doi: 10.1046/j.1523-1755.2002.00204.x. [DOI] [PubMed] [Google Scholar]
- 60.Baumann M, Sollinger D, Roos M, Lutz J, Heemann U. Prehypertensive preconditioning improves adult antihypertensive and cardioprotective treatment. J Pharmacol Exp Ther. 2010;332(3):1121–6. doi: 10.1124/jpet.109.161075. [DOI] [PubMed] [Google Scholar]
- 61.Toran-Allerand CD, Guan Xiaoping, MacLusky Neil J, Horvath Tamas L, Diano Sabrina, Singh Meharvan, et al. ER-X: A Novel, Plasma Membrane-Associated, Putative Estrogen Receptor That Is Regulated during Development and after Ischemic Brain Injury. J Neuroscience. 2002;22(19):8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratziu V, Zelber-Sagi S. Pharmacologic therapy of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):667–88. doi: 10.1016/j.cld.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Kulke MH, Chan JA, Meyerhardt JA, Zhu AX, Abrams TA, Blaszkowsky LS, Regan E, Sidor C, Fuchs CS. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68(2):293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5(1):22–7. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 65.Dantas A, Sandberg Kathryn. Does 2-Methoxyestradiol represent the new and improved Hormone replacement Therapy for Atherosclerosis? Circulation Research, AHA. 2006;99:234–7. doi: 10.1161/01.RES.0000236802.00855.cd. [DOI] [PubMed] [Google Scholar]
- 66.Brueggemeier RW, Bhat Abhijit S, Lovely Carl J, Oughenour Holly D, Joomprabutra Surachai, Weitzel Douglas H, et al. 2-Methoxymethylestradiol: a new 2-methoxy estrogen analog that exhibits antiproliferative activity and alters tubulin dynamics. Jour Steroid Biochem and Mol Biol. 2001;78(2):145–156. doi: 10.1016/s0960-0760(01)00090-5. [DOI] [PubMed] [Google Scholar]
- 67.Gui Y, Zheng XL, Zheng J, Walsh MP. Inhibition of rat aortic smooth muscle contraction by 2-methoxyestradiol. Am J Physiol Heart Circ Physiol. 2008;295(5):H1935–42. doi: 10.1152/ajpheart.00723.2008. [DOI] [PubMed] [Google Scholar]
- 68.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 69.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34(4 Pt 2):931–6. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- 70.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280(1):C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 71.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endiothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 72.Roberts JM, Lain KY. Preterm birth and pre-eclampsia bad news and good news. Lancet. 1998;352:SIV22. [PubMed] [Google Scholar]
- 73.Gerretsen G, Hiusjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 74.Duffy AA, Martin MM, Elton TS. Transcriptional regulation of the AT1 receptor gene in immortalized human trophoblast cells. Biochim Biophys Acta. 2004;1680(3):158–70. doi: 10.1016/j.bbaexp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Schmid M, Sollwedel A, Thuere C, Wafula PO, Zenclussen ML, Müller DN, Gratze P, Woiciechowsky C, Volk HD, Zenclussen AC. Murine pre-eclampsia induced by unspecific activation of the immune system correlates with alterations in the eNOS and AT1 receptor expression in the kidneys and placenta. Placenta. 2007;28(7):688–700. doi: 10.1016/j.placenta.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Itoh T, Kajikuri J, Tada T, Suzuki Y, Mabuchi Y. Angiotensin II-induced modulation of endothelium-dependent relaxation in rabbit mesenteric resistance arteries. J Physiol. 2003;548(3):893–906. doi: 10.1113/jphysiol.2002.034116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 78.Dubey RK, Tofovic Stevan P, Jackson Edwin K. Cardiovascular Pharmacology of Estradiol Metabolites. JPET. 2004;308(2):403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 79.Prossnitz ER, Arterburn Jeffrey B, Sklar Larry A. GPR30: A G protein-coupled receptor for estrogen. Molecular and cellular Endocrinology. 2007;265–266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 81.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 82.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 83.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 84.Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackson EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension. 2000;35(1 Pt 2):262–6. doi: 10.1161/01.hyp.35.1.262. [DOI] [PubMed] [Google Scholar]