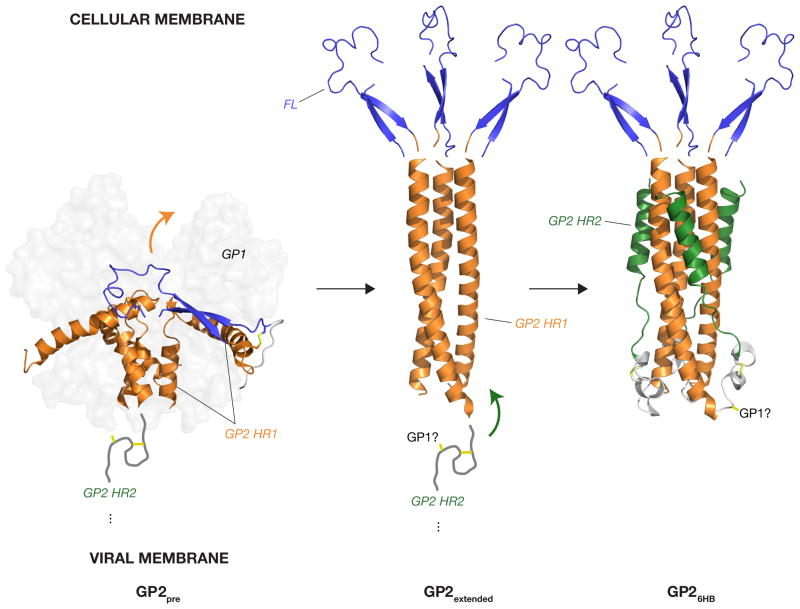
Fig. 2. Proposed structural rearrangements in GP2 during entry.
In response to the fusion trigger, the HR1 sequence in the pre-fusion GP2 conformer (GP2pre) is proposed to undergo release from the GP1 base subdomain and to rearrange to an unbroken α–helix, projecting the GP2 fusion loop (FL) into the target membrane. This rearrangement is mediated by a conformational transition in HR1 . The resulting extended GP2 conformation tipped by fusion loops is then thought to rearrange further, with the sequence betwen HR1 and HR2 reversing direction, and the HR2 sequences packing against grooves in the trimeric HR1 coiled-coil to form the six-helix bundle (6HB) (GP26HB). Orange and green arrows show the direction of motion of HR1 and HR2, respectively. Structures from PDB ids: 3CSY [9] and 1EBO [7]. Fusion loops in GP2extended and GP26HB are derived from the GP pre-fusion structure 3CSY, and their positions are indicated for illustration only. GP2extended is hypothetical. Only one of three GP2 fusion loops in 3CSY is shown for clarity. A cartoon of the GP2 sequence between the HR1 and HR2 with GP1-GP2 and GP2-GP2 disulfide bonds shown (yellow sticks) is based on the structure of the Sudan virus GP pre-fusion trimer (3S88 [10].