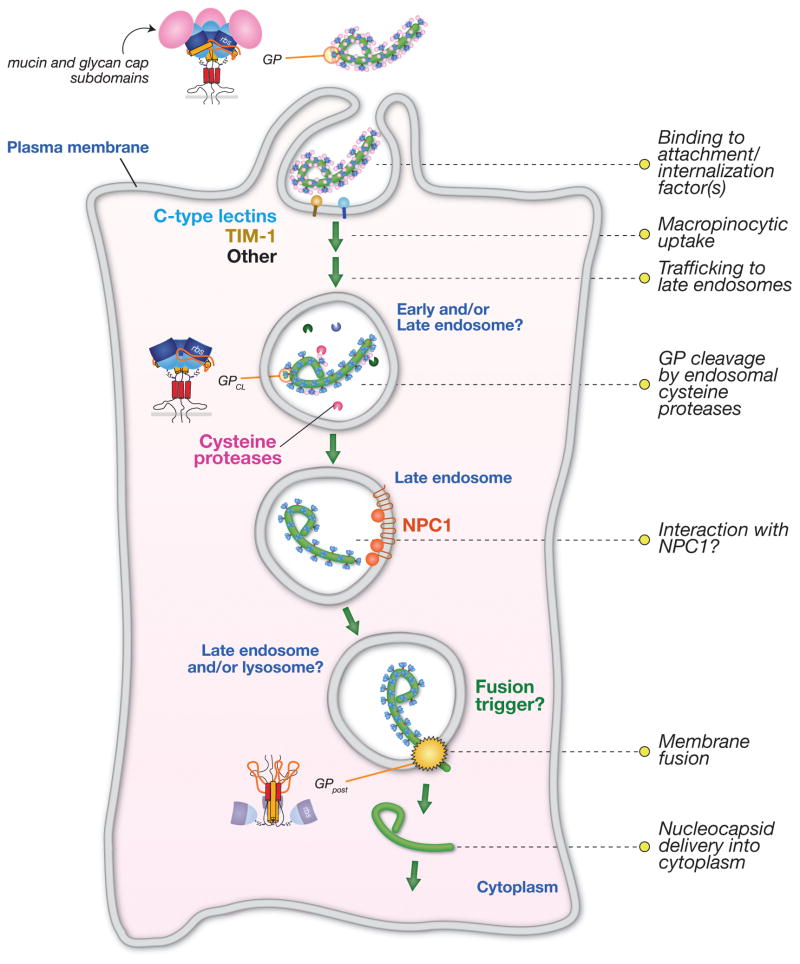
Fig. 3. Schematic model of filovirus entry pathway.
The filovirus glycoprotein, GP, mediates viral attachment to cells via multiple and at least partly redundant interactions with cell-surface molecules. GP then induces viral internalization through a macropinocytosis-like pathway. Within the endocytic pathway, GP is cleaved by endosomal cysteine proteases to generate a primed intermediate that resembles GPCL derived by in vitro proteolysis. Viral particles are delivered to late endosomal/lysosomal compartments, where cleaved GP may interact with NPC1, which plays a critical role at one or more late steps in filovirus entry. It is currently not known if NPC1 is the longsought endosomal receptor or if another host molecule plays this role. An unknown host trigger then induces rearrangements in cleaved GP that drive viral membrane fusion and cytoplasmic escape of the viral nucleocapsid core. The subcellular location of viral membrane fusion has not been defined, but late endosomes and/or late endosome-lysosome hybrids are plausible candidates.