Abstract
Anthrax lethal toxin (LT), a major virulence determinant of anthrax disease, induces vascular collapse in mice and rats. LT activates the Nlrp1 inflammasome in macrophages and dendritic cells, resulting in caspase-1 activation, IL-1β and IL-18 maturation and a rapid cell death (pyroptosis). This review presents the current understanding of LT-induced activation of Nlrp1 in cells, and its consequences for toxin-mediated effects in rodent toxin and spore challenge models.
Keywords: anthrax lethal toxin, inflammasome, caspase-1, Nlrp1, Nlrp1b, macrophage, interleukin-1, Bacillus anthracis
1. Background
Anthrax disease results from introduction of Bacillus anthracis spores into host organisms by inhalation, ingestion, or penetration of the skin. It requires spores to germinate and to produce toxins that enable bacterial dissemination. B. anthracis produces three proteins that make up anthrax lethal toxin (LT) and edema toxin (ET). Protective antigen (PA), the receptor binding component common to both toxins, delivers lethal factor (LF), a protease [1], or edema factor (EF), an adenyl cyclase [2], into cells. The vascular collapse associated with anthrax infection can be largely replicated in animals by challenge with the toxins alone [3,4,5,6], and immunization against PA is sufficient for protection from infection (for review see [7]). Animal infections using unencapsulated bacteria in which the toxin component genes have been knocked out show that while both toxins play a role in dissemination [8,9], it is principally LT which is responsible for the lethality caused by anthrax [10,8].
The only substrates known to be cleaved by LF are the mitogen-activated protein kinase kinases (MAPKKs, MKKs, or MEKs) [11,12,13,14]. Although the MAPK (ERK)1/2, JNK/SAPK, and p38 signaling pathways disrupted by this toxin are essential to many cellular functions, a link between their shutdown and LT's induction of lethality has not been found. Furthermore, the rapid lysis which LT induces in sensitive macrophages from certain inbred mice [15,16] and rats [17,18] requires more than the cleavage of the MEK substrates, because this cleavage also occurs in macrophages resistant to LT-mediated lysis [14,19]. LF targeting of the MEK pathways in cells that control the innate immune response, however, plays a role in disabling their ability to battle infection, thereby aiding bacterial dissemination and establishment of disease [20,8].
LT induces an atypical cytokine-independent vascular collapse in rodents, which differs greatly from the effects induced by ET, in that it is not accompanied by hemorrhaging lesions or fibrin deposition [3,4]. There are almost no classic shock-associated histopathological changes found in mice or rats challenged with LT, and the tissue necrosis that is observed appears to result from the hypoxia that follows vascular collapse [4]. Inbred mice succumb to LT over a period of days and can display a wide range of sensitivities which are independent of their macrophages' sensitivity to LT [21]. In contrast, when challenged with saturating doses of toxin, rats succumb in less than one hour, and as rapidly as 37 minutes [22,23,24]. Rat death is a dichotomous phenotype, with inbred strains divided on the basis of absolute sensitivity or resistance to LT [18]. Rapid hemodynamic changes are observed in LT-challenged rats [3,25,26], but the shock induced by LT differs from endotoxin-induced shock and is resistant to standard therapies [26,27]. The rapidity of rat death suggests toxin-induced molecular events that result in heart failure and an associated pulmonary edema [28,29,30]. Interestingly, in a perfused rat heart model, LT had no significant effect at doses at which ET produced rapid functional changes [31]. The heart is also one of the earliest organs targeted in mice, although it is unclear if LT's effects on the heart are direct or result indirectly from targeting of the vasculature at distant sites [32]. Remarkably high levels of cardiac biomarkers are observed in the blood of mice as early as 6–8 hours after toxin treatment [32]. While the sensitivity of mice to anthrax toxin appears to involve multiple genetic factors ([33,21] and our unpublished work), the sensitivity of rats to rapid toxin-induced death was recently mapped to a limited number of polymorphisms present in the first 100 amino acids of the inflammasome sensor Nlrp1 [18].
2. The rodent Nlrp1 inflammasomes
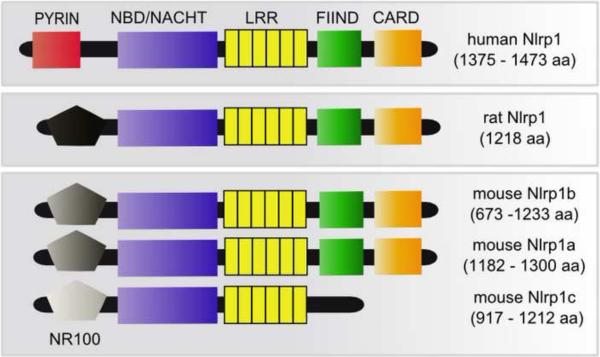
Inflammasomes are multimeric protein complexes which form in response to cytoplasmic danger signals and provide a scaffold for the activation of caspase-1. Inflammasomes contain a sensor NLR (Nod-like receptor/nucleotide-binding domain leucine-rich repeat containing protein) component (see Figure 1) which undergoes a conformational change in response to a danger signal and recruits inactive pro-caspase-1. The inflammasome complex then mediates caspase-1 activation, and active caspase-1 processes proinflammatory cytokines IL-1β and IL-18 to their mature forms, allowing for their rapid secretion from the cell. In macrophages and dendritic cells, active caspase-1 also targets death substrates which have yet to be identified, thereby inducing a rapid (1–3 hour) cell death termed pyroptosis. There are numerous NLRs which sense different pathogen or danger-associated molecular patterns as well as environmental stimuli. Some of the best studied NLRs include Nlrp3/Nalp3/Cryopyrin/CIAS1/PYPAF1 (which recognizes fungi, intracellular bacteria, pore-forming toxins, extracellular ATP, and crystalline material such as silica, urea crystals, asbestos, amyloid-β, and alum), Nlrc4/IPAF (which recognizes Gram-negative bacteria with type III or type IV secretion systems and flagellin), and AIM2 (recognizing dsDNA and DNA viruses) (for review see [34]). Most NLR proteins associate with the scaffold protein ASC (apoptosis-associated speck-like) via a pyrin domain and once caspase-1 is recruited, this association with ASC is required for the inflammasome's cytokine processing function [35]. Caspase-1 induction of pyroptosis, however, does not require ASC [35]. While human Nlrp1 contains an N-terminal pyrin domain through which it associates with ASC, rodent Nlrp1 proteins are missing pyrin domains in their N-termini, and instead contain a region with little to no homology to other NLRs (Figure 1). We have provisionally named this unique domain “NR100” (N-terminal domain of rodent Nlrp1 proteins, approximately 100 amino acids) (Figure 1).
Figure 1.
Schematic overview of the domains of the intracellular sensor Nlrp1 from human, rat, and mouse. The genomes of mice contain three Nlrp1 paralogs on chromosome 11 (Nlrp1a, Nlrp1b, and Nlrp1c) and different mouse strains express two or three of these proteins, as well as different splice variants. Human Nlrp1 contains an ASC-interacting pyrin domain that is absent in rodent Nlrp1s. Rodent Nlrp1s have a unique N-terminal region (NR100, for “N-terminal domain of rodent Nlrp1of about 100 amino acids”) with no sequence homology to human Nlrp1 and limited homology between the rodent species and paralogs, as indicated by shading in the figure. Other domains shown are: NBD/NACHT, nucleotide binding ATPase domain; LRR, leucine-rich repeats; FIIND, function-to-find domain; CARD, caspase-1 recruitment domain. Please note that the relative size of domains is not to scale, and the number of LRR domain repeats differs based on species and paralog and can also vary depending on the algorithms used to assign them.
In mice, three tandem Nlrp1 genes (Nlrp1a, Nlrp1b and Nlrp1c) are present on chromosome 11 [36]. Sensitivity or resistance of mouse macrophages from different inbred strains to LT-induced pyroptosis is controlled by Nlrp1b, and sensitivity is a dominant trait [36]. In rats, there are two paralogs, but only one (Nlrp1) appears to be expressed in most strains and this protein also controls LT-induced macrophage pyroptosis [18]. Interestingly, in rats the Nlrp1 locus may also control the rapid 37-minute animal death induced by LT [18]. To date, the only known activator of rodent Nlrp1 is LT and there are no known in vivo activators of human Nlrp1, although activation by the bacterial ligand muramyl-dipeptide has been reported in studies with an in vitro reconstituted inflammasome [37,38].
In the following sections we review the current knowledge of the role of the Nlrp1 inflammasome in mouse and rat macrophage death, animal susceptibility to LT, and susceptibility to spore infection.
2.1 The Nlrp1 inflammasome and cell death
2.1.1 Nlrp1 and LT-induced rodent macrophage pyroptosis
Macrophages and dendritic cells from inbred mice and rats exhibit either absolute sensitivity or resistance to the rapid pyroptosis induced by LT. While pyroptosis-resistant macrophages and dendritic cells from both rodents succumb to caspase-1-independent apoptosis after longer periods (>16 hours) of toxin treatment [39,40], sensitive mouse macrophages lyse 60–90 minutes after treatment with saturating toxin concentrations, and sensitive rat macrophages require 120–150 minutes for lysis [41]. Despite the slightly slower pyroptosis of rat macrophages, the Nlrp1- and caspase-1-dependent mechanism of cell death appears to be highly similar in mice and rat cells [41,42]. This cell death requires both caspase-1 activation and expression of certain Nlrp1 alleles associated with macrophage sensitivity to LT (Nlrp1bS) [36,43,39,41,42].
Nlrp1b was identified as the candidate Nlrp1 paralog for controlling sensitivity to LT in mouse macrophages as it is the only paralog expressed in all the 129-lineage mouse strains that harbor LT-sensitive macrophages ([36] and data not shown). In the original report identifying this gene as the LT sensitivity locus, expression of Nlrp1bS in mice also harboring a resistant Nlrp1b allele (Nlrp1bR) was shown to confer sensitivity to LT, and inhibition of Nlrp1bS expression with interfering RNA was protective [36]. Sequencing of the locus in a large panel of inbred mice identified five polymorphic Nlrp1b alleles, two conferring sensitivity and three conferring resistance to LT. However, comparisons of these alleles did not allow for a correlation of sensitivity with particular amino acid polymorphisms [36]. Interestingly, the Nlrp1bS in the CAST/EiJ strain is highly similar in sequence to the Nlrp1bR present in the NOD/ShiLtJ and AKR/J mice. Only sixteen amino acid differences exist between these Nlrp1bS and Nlrp1bR proteins, offering an opportunity to dissect the structural basis of sensitivity and resistance. However, the variations in expression of Nlrp1 paralogs in various inbred strains and the existence of multiple splice variants complicate the analyses of the role Nlrp1b or its particular domains play in sensitivity to LT in mice.
In rats, the only expressed Nlrp1 gene is remarkably conserved across all strains [18], and sequence and phenotypic analyses in 12 rat strains identified two Nlrp1S and three Nlrp1R alleles. Comparison of these resistant and sensitive Nlrp1 proteins showed a perfect correlation between the polymorphisms present in the first 100 residues of the protein and LT sensitivity [18]. This region of Nlrp1 does not contain any recognized functional domains (Figure 1). The mechanism(s) through which these polymorphisms control the rat Nlrp1 response to LT is currently unknown, and even more perplexing is the absence of any sequence similarity between the key 100-amino acid region of the rat Nlrp1S and any portion of the mouse Nlrp1b proteins. Although the N-terminal region of rodent Nlrp1 has no recognized domain structure and no known function, the corresponding region of human Nlrp1 contains a pyrin domain that is responsible for binding to the inflammasome adaptor ASC. The absence of a pyrin domain at the N-terminus of mouse and rat Nlrp1 is consistent with the observation that the LT-induced Nlrp1bS inflammasome complex in macrophages appears to lack ASC [44]. However, it has been recently reported that while ASC is necessary for caspase-1 autoproteolytic activation and efficient cytokine processing, inflammasomes that function independently of ASC induce caspase-1 activation and pyroptosis even in the absence of caspase-1 autoproteolysis [35]. LT was used as an inducer of caspase-1 autoproteolysis-independent pyroptosis in these studies. However, caspase-1 always undergoes autorproteolysis in response to LT [45,46,43,41,47,36]. Thus it may be possible that two distinct Nlrp1 inflammasomes form in response to the toxin, one of which contains ASC or a yet unknown scaffold leading to caspase-1 autoproteolysis and cytokine processing, and a separate ASC-deficient inflammasome which results in induction of pyroptosis.
Interestingly, an inflammasome reconstitution study in fibroblasts has suggested that Nlrp1bS and caspase-1 alone are sufficient to form an LT-responsive inflammasome which cleaves IL-1β and results in a modest degree of fibroblast death in response to the toxin [48]. The killing of fibroblasts by LT implies that the NADPH oxidase activity and reactive oxygen species limited to macrophages and previously suggested to be essential to LT-mediated pyroptosis [49], are not absolutely required for the toxin's induction of cell death. These results also suggest that any tissues in which Nlrp1 and caspase-1 are expressed in sufficient quantity should be susceptible to pyroptosis.
As is the case for most NLRs and inflammasomes, little is known about the actual signal or mechanism by which LT activates rodent Nlrp1 proteins. Similar to other well-characterized inflammasomes [50], potassium efflux is required for activation of rodent Nlrp1 inflammasomes [43,51], and this event appears to be upstream of caspase-1 cleavage. LT has recently been reported to enhance activity of both Kir and Kv potassium channels in mouse macrophages independent of Nlrp1b sequence and caspase-1 activation [52]. Expression of a dominant negative form of Kir in Nlrp1bS-expressing macrophages blocked the LT-induced caspase-1 activation and subsequent IL-1β release, supporting a direct link between potassium channel function and toxin-mediated Nlrp1b activation [52]. However, since potassium efflux is a common requirement for activation of other NLR sensors [50], it is unclear why the Nlrp1b-independent alteration in channel function would not lead to activation of a number of NLR sensors in an LT-independent fashion. Thus, it appears that potassium efflux alone is not sufficient as a signal for LT's activation of the Nlrp1 inflammasome.
A unique trait of LT-induced activation of Nlrp1 is its susceptibility to inhibition by proteasome inhibitors [43,53,51,41]. This inhibition is independent of LF's proteolytic action on MEK substrates and can be achieved even with very late addition of these inhibitors, as long as caspase-1 activation has not progressed [43,53,51,54]. It is possible that one or more host proteins that could function either as Nlrp1 inhibitors or as LT substrates must be broken down by the proteasome for Nlrp1 activation. It is likely that the putative target of the proteasome in LT-treated macrophages is subject to the N-end rule of protein breakdown [55], because type-2 destabilizing amino acid derivative inhibitors of this pathway such as phenylalanine amide also protect against LT [56]. The only currently identified N-end rule substrate targeted for proteasome breakdown by LT is cIAP1, a member of the inhibitor of apoptosis protein (IAP) family [56]. Proteasome-mediated breakdown of the IAP family of proteins has been associated with other pathways of cell death, but not with pyroptosis; thus, a link between this protein and caspase-1-dependent cell death has not yet been demonstrated.
Other proteins and pathways have also been implicated in LT-induced, Nlrp1-dependent cell death at steps that are downstream of toxin entry and cleavage of the MEK substrates. For example, Nlrp1bS -harboring macrophages having reduced expression of the mitochondrial proteins Bnip3 and Bnip3L (Bcl2/adenovirus E1B-interacting proteins 3 and 3L) are resistant to LT, while Nlrp1bR-harboring macrophages in which these proteins are overexpressed are sensitized to the toxin [57]. This suggests involvement of mitochondrial-associated events, as Bnip3 has been implicated in death pathways involving mitochondrial membrane permeabilization, ROS production and rapid changes in plasma membrane integrity [58] and LT also induces similar changes in macrophages [54]. Recently, AMP deaminase 3, which converts AMP to IMP, was also implicated in LT-induced pyroptosis [59]. Knockout of the gene in macrophages harboring an Nlrp1bS allele results in resistance to LT-induced pyroptosis, but its role in caspase-1 activation or possibly in protective mechanisms occurring downstream of caspase-1 is unknown.
A number of other protective treatments have been reported to inhibit LT-mediated activation of the Nlrp1 inflammasome, and the steps at which they function have been elucidated. Examples include reports showing a heat shock-induced trapping of pro-caspase-1 in a large complex, therefore preventing its autoproteolytic activation [45], celesterol-induced inhibition of proteasome activity [60], auranofin-mediated inhibition of caspase-1 activity [47], as well as a role of idebenone, which acts as an inhibitor of potassium channels, and synergizes with auranofin for increased protection [47]. Inhibition of cathepsin B activity with drugs such as CA-074Me can also inhibit LT's activation of the Nlrp1 inflammasome and cell death [61,46]. However it is unclear whether cathepsin B acts upstream of caspase-1 activation or is part of a feedback loop that requires active caspase-1. In either case, the lysosomal membrane permeabilization that leads to increases in cytoplasmic cathepsin B activity is a hallmark of Nlrp1S-dependent pyroptosis, much in the manner observed for other inflammasomes [61,46]. It should be noted that cathepsin B has also been suggested to play a different role in delivery of LF to the cytosol following endosome-lysosome fusion and autophagic flux [62].
2.1.2 Nlrp1 and LT targeting of MEK pathways
The only identified substrates for LF are MEK 1, 2, 3, 4, 6 and 7. The cleavage of these kinases by LF leads to the inactivation of the ERK1/2, p38, and SAPK/JNK signaling pathways. However, no link has been found between LF's action on these substrates and the toxin's activation of the Nlrp1 inflammasome, and MEK substrates are cleaved in all cells independent of their sensitivity to pyroptosis. It is however possible that the cleavage of the MEK proteins is sensed differentially by the Nlrp1 proteins in sensitive and resistant macrophages. Early work suggested that in macrophages, LF-mediated inhibition of the p38 pathway results in cell death; however, these findings related to the delayed apoptosis induced by LF that occurs in cells expressing either Nlrp1bS or Nlrp1bR [63]. In a more recent report, the same investigators used MEK6 constructs that are resistant to LF-mediated cleavage to suggest that inhibition of p38 is the primary mechanism by which cell death is induced in B. anthracis-infected macrophages. This study indicated that cell death induced by infection with LT-expressing bacteria led to ATP leakage which was the cause of a “bystander” activation of the Nlrp1b inflammasome [64]. Perplexingly, all the experiments in this report were performed in cells isolated from C57BL/6J mice and knockout strains, all harboring the Nlrp1bR protein which is LT-nonresponsive. Adding another level of complexity is that these studies used bacterial infections with B. anthracis strains which express LF or are mutated for its production, and it is highly likely the bacteria provide numerous other stimuli capable of activating other inflammasomes. It is therefore possible that many observations in this study result from the activation of Nlrp3 or other inflammasomes by bacterial infection, and that the expression of LF, through targeting of the MEK pathways, is simply modulating the infected cell's ability to function. Furthermore, previous studies using more specific inhibitors of the P2X7 receptor eliminated ATP leakage as the mechanism for the rapid cell death caused by LT [65]. LT-treated Nlrp1bS-macrophages have no paracrine effect on Nlrp1bR macrophages even when growing on or with membrane-to-membrane contact, and ATP hydrolyzing enzymes have no protective effect against LT (Moayeri et al. unpublished).
Another recent report investigating the link between the inactivation of MEK pathways and pyroptosis used a different approach. An LF mutant which was defective in its ability to induce pyroptosis in Nlrp1bS-expressing macrophages was isolated [66]. This mutant toxin could not activate the inflammasome (as measured by IL-1β cleavage) in a reconstituted system where Nlrp1bS, caspase-1, and IL-1β were expressed in fibroblasts, but it still inhibited the MEK 1/2/ERK signaling pathways as efficiently as wild type toxin. However, the LF mutant was inefficient in cleavage of MEK3/6 and thus not able to prevent activation of the p38 pathway, suggesting that the MEK3/6 pathway provides a protective mechanism against pyroptosis and that inactivation of this pathway may contribute to inflammasome-induced death. Alternatively, it is possible the mutant LF was defective in cleavage of an unknown substrate required for inflammasome-mediated cell death.
2.1.3 Outstanding questions
Although a limited number of polymorphisms in the N-terminus of rat Nlrp1S are sufficient to determine whether the protein is activated by LT, the same polymorphisms are not present in any mouse Nlrp1bS proteins – and yet both mouse and rat Nlrp1 are activated in response to LT, resulting in a similar pyroptosis. It is unclear how LT activates the inflammasome, or how caspase-1 activation leads to cell death. MEK cleavage by LF, which occurs equally in sensitive and resistant macrophages [14,19], has not yet been definitively linked to the caspase-1-induced events required for pyroptosis.
2.2 Nlrp1 and LT-induced rodent death
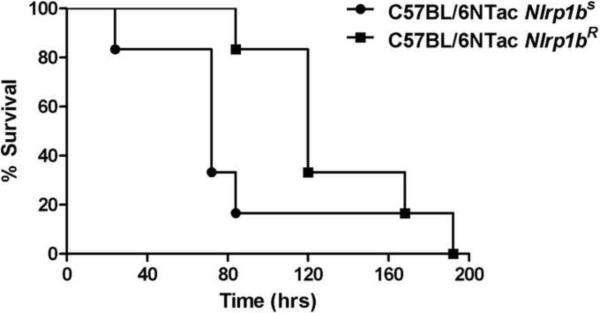
Inbred mice display a wide range of sensitivities to LT challenge, with some strains being resistant (NOD/ShiLtJ; DBA/2J) and others highly sensitive (Balb/cJ; CAST/EiJ) [67]. Injection of LT into mice that harbor Nlrp1bS alleles leads to Nlrp1b activation, macrophage lysis, and release of large amounts of IL-1β within 1–2 hours after injection [4,21]. Inflammasome activation by LT in vivo and the associated remarkable IL-1β release do not require a second, e.g., TLR-mediated signal, as seen for other inflammasomes. Furthermore, we find that congenic C57BL/6NTac mice harboring two Nlrp1bS alleles always succumb to LT 36–48 hours earlier than mice harboring two Nlrp1bR alleles (Figure 2). These mice are similar at >98% of all other genomic loci, indicating that the IL-β release that rapidly results in an extensive cytokine storm in mice harboring Nlrp1bS alleles may exacerbate LT-mediated vascular collapse, though it is not necessary for LT-induced lethality [4,21]. Accordingly, inhibition of macrophage lysis in LT-treated Balb/cJ (Nlrp1bS) mice by proteasome inhibitors increases survival time [42]. However, it should be noted that some studies have not observed a more rapid death of LT-challenged transgenic animals in which Nlrp1bS is expressed in a resistant background [68]. Whether the Nlrp1bS dependent cytokine storm accelerates the toxin's effects in mice or not, the vascular shock LT induces is not dependent on macrophage lysis [4,21,68]. Further supporting the conclusion that Nlrp1-mediated macrophage pyroptosis is not required for the mouse lethality induced by LT is the fact that myeloid-lineage specific anthrax toxin receptor-deficient mice [8] and macrophage-deficient mice [21] are also fully sensitive to LT challenge. Thus, it appears that other genetic loci control animal sensitivity to toxin. These may include proteins encoded by multiple loci on mouse chromosome 11 [33] or genes linked to the murine endocrine system, which has been shown to have a striking contribution to the lethality induced by LT [69].
Figure 2.
Mice (n=11/group) were injected with PA (100 μg) + LF (100 μg) intraperitoneally and monitored for survival for ten days.
In contrast to mice, rats do not exhibit intermediate sensitivities to LT and either succumb to saturating doses of LT in under one hour or they are completely resistant. The LT dose (10 µg) that kills a sensitive 200-g rat in 60 minutes has little effect on an LT-sensitive 20-g mouse. Thus, despite a similar vascular collapse event accompanied by cardiac failure, LT-induced death occurs with very different timing and dose requirements in mice and rats, and therefore may involve different tissue and cell targets.
In rats, a perfect correlation between macrophage sensitivity and rat susceptibility to LT is seen among all tested inbred strains as well as among recombinant inbred rats from the HXB/BXH collection [18]. This collection, developed by two gender-reciprocal matings of the SHR/Ola rat (LT-resistant) with the BN-Lx rat (LT-sensitive), allowed mapping of rat sensitivity to toxin-induced death to a region of chromosome 10 containing Nlrp1. Surprisingly, the N-terminal sequence polymorphisms associated with LT-sensitive macrophages (as described in the previous section) also correlate perfectly with rat susceptibility to death. Furthermore, proteasome inhibitors which prevent caspase-1 activation by LT can delay or protect against toxin-induced rat death [42,41]. Thus Nlrp1 is the primary candidate for conferring rat sensitivity to LT. However, it is unlikely that toxin-induced pyroptosis or IL-1β/IL-18 signaling plays an important role in LT-mediated rat death. For example, we have found that macrophages from heterozygous rats harboring one sensitive and one resistant allele of Nlrp1 require >8 hours to die in response to LT, while the animals themselves succumb in 1 hour. Furthermore, bone marrow chimera studies, cell transfusions, macrophage depletion analyses, anakinra (IL-1 receptor antagonist) treatment, and neutralizing IL-1β/IL-18 studies demonstrate that irradiation-sensitive myeloid cells and IL-1β/IL-18 signaling do not play a role in the rat death caused by LT (Moayeri et al., unpublished). Thus, activation of Nlrp1 in other, unidentified cells and tissues appears to play a key role in causing toxin-induced rat death. In contrast to the earlier view that NLR sensors and caspase-1 function primarily in myeloid, first responder innate immune cells, it is now recognized that NLR proteins also have roles in a variety of non-myeloid tissues [70]. The large number of caspase-1 substrates that have been identified [71] is consistent with the view that this protease plays an important role in many different cell types. Of course the possibility cannot be excluded that rat susceptibility to LT is controlled by a different gene closely linked with Nlrp1 on chromosome 10 that does not segregate at a detectable frequency. A similar situation exists in mice, where almost all polymorphisms in the Kif1c gene, which is co-inherited with the Nlrp1a-b-c locus, also correlate with the Nlrp1b polymorphisms that are associated with LT susceptibility [21,72]. However, the likelihood is low that LF targets two different proteins encoded by adjacent genes on the chromosome, one controlling macrophage lysis and the other animal death. Thus, we believe that it is Nlrp1 which is controlling LT-mediated rat death through unknown mechanisms that involve yet to be identified target tissues.
2.3 Nlrp1 and anthrax infection
Early studies hypothesized that macrophages act as a vessel for the dissemination of anthrax spores and bacteria [73], and later studies suggested that anthrax toxins play a role in allowing bacteria to survive the macrophage's phagocytic and bacteriocidal activities [74,75]. More recently, mouse genetics has provided indisputable evidence on the role of LT in battling the host innate immune response [8]. Nlrp1bR mice in which anthrax toxin receptors were knocked out only in the myeloid cell population were made more resistant to anthrax infection, strongly arguing that MEK pathways essential to macrophage and/or neutrophil function are targeted by LT as a key step during establishment of anthrax infections [8].
It is not surprising that macrophages play a role in protection against infection independent of the Nlrp1 response to LT. However, the differential response of Nlrp1bS and Nlrp1bR expressing macrophages to LT has a separate, potent effect on infection in the mice harboring them. The basis for the effect of Nlrp1b status on infection was actually observed many years before the gene was identified. Early studies noted the surprising finding that macrophage sensitivity to LT (and even animal sensitivity to LT for some species) was inversely correlated with susceptibility to anthrax infection [76,77]. These findings would suggest that LT-mediated pyroptosis is actually detrimental to establishing infection. Recent studies using large panels of inbred strains, as well as Nlrp1b congenic/transgenic mice (described in the previous section) have allowed a better dissection of this inverse relationship [68,78]. Mice harboring Nlrp1bS alleles are resistant to infection at doses at which Nlrp1bR counterparts succumb [68,78]. The protection is independent of infection route or spore germination, and is amazingly potent in that it acts even against injection of large numbers of vegetative bacteria directly into the bloodstream [78]. The Nlrp1bS allele can no longer impart resistance when caspase-1 and IL-1 receptor are knocked out in mice harboring the allele, suggesting it is likely activation and release of IL-1β/IL-18 in an Nlrp1bS-dependent fashion is crucial to protection against spore infection [78]. The IL-1 receptor antagonist anakinra also reverses Nlrp1bS-mediated resistance in mice, supporting a role for caspase-1-dependent IL-1 signaling in protection [78]. It is highly likely that the differential sensitivity of Nlrp1bS- and Nlrp1bR-harboring mice to infection is due to differences in the response of these proteins to LT, although the possibility does exist that other bacterial components are sensed by Nlrp1bS. However, the higher IL-1β and resultant circulatory neutrophil levels seen in Nlrp1bS mice in response to LT are likely linked to the mechanism through which this inflammasome sensor ultimately contributes to resistance to infection [78].
Surprisingly, in rats, Nlrp1S-mediated control of anthrax infection is not seen (Moayeri et al., unpublished). Studies using six different inbred rats show that although rats are generally extremely resistant to anthrax infection, when they do succumb it is in accordance to their toxin sensitivity (and thus, Nlrp1 status) (Moayeri et al., unpublished). The IL-1β/IL-18 response to LT challenge as well as anthrax infection in Nlrp1S rats is extremely low and may explain the absence of protection in rats (Moayeri et al., unpublished). Interestingly, there is precedent for these findings. LT challenges studies performed in the 1960s showed striking differences between the NIH black (Nlrp1R) and Fischer (Nlrp1S) rats [79,77]. Although the NIH black rat was very resistant to toxin treatment, it was highly sensitive to bacterial dissemination during B. anthracis infections, and far higher numbers of bacteria were measured in its bloodstream than in that of similarly infected Fischer (Nlrp1S) rats. However, despite the lower bacterial load in the highly LT-sensitive Fischer rat, these animals died more rapidly from the infection, much in the manner observed in our current studies [79,77]. Investigators interpreted their findings as reflecting two phases of anthrax disease, a first battle to establish infection, followed by succumbing to toxins later in disease. In rats, sensitivity to toxin-mediated death appears to be more important than any role Nlrp1 may play in establishing infection.
3. Concluding remarks: What about anthrax infection in humans?
Having discussed the role of Nlrp1 in mice and rats, it must be asked how the insights gained from these experimental animal models might apply to anthrax disease in humans. To date, all macrophages isolated from human volunteers and tested in our laboratory and many others have been found to be resistant to LT. As mentioned before, human Nlrp1 differs substantially from rodent Nlrp1 proteins in that it harbors an N-terminal pyrin domain, and this can be expected to confer responsiveness to LT that differs from that of rodent Nlrp1 proteins. In any case, the apparent “resistance” of the human Nlrp1 allele could imply that humans are among the species having higher susceptibility to anthrax infection, much in the manner of Nlrp1bR mice, since the potent and potentially protective LT-induced, Nlrp1bS-dependent cytokine responses are absent. It remains to be seen if human Nlrp1 responds to anthrax infection in a manner independent of LT. In any event, the studies on the very interesting rodent Nlrp1 proteins which were discussed above provide useful information about the nature of inflammasome activation and caspase-1 action in general, and this are likely to have value in understanding the innate immune response to infectious diseases in humans.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Klimpel KR, Arora N, Leppla SH. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 1994;13:1093–1100. doi: 10.1111/j.1365-2958.1994.tb00500.x. [DOI] [PubMed] [Google Scholar]
- [2].Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am. J. Physiol. Regul. Integr. Comp Physiol. 2004;286:R699–R709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- [4].Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-á-independent hypoxia-mediated toxicity in mice. J. Clin. Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beall FA, Dalldorf FG. The pathogenesis of the lethal effect of anthrax toxin in the rat. J. Infect. Dis. 1966;116:377–389. doi: 10.1093/infdis/116.3.377. [DOI] [PubMed] [Google Scholar]
- [6].Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang WJ, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chitlaru T, Altboum Z, Reuveny S, Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol. Rev. 2011;239:221–236. doi: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- [8].Liu S, Miller-Randolph S, Crown D, Moayeri M, Sastalla I, Okugawa S, Leppla SH. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. 2010;8:455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dumetz F, Jouvion G, Khun H, Glomski IJ, Corre JP, Rougeaux C, Tang WJ, Mock M, Huerre M, Goossens PL. Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax. Am. J. Pathol. 2011;178:2523–2535. doi: 10.1016/j.ajpath.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- [12].Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- [13].Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- [14].Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- [15].Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- [16].Friedlander AM, Bhatnagar R, Leppla SH, Johnson L, Singh Y. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 1993;61:245–252. doi: 10.1128/iai.61.1.245-252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nye SH, Wittenburg AL, Evans DL, O'connor JA, Roman RJ, Jacob HJ. Rat survival to anthrax lethal toxin is likely controlled by a single gene. Pharmacogenomics J. 2007;8:16–22. doi: 10.1038/sj.tpj.6500448. [DOI] [PubMed] [Google Scholar]
- [18].Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS. Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Lethal factor of Bacillus anthracis cleaves the N-terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 2000;290:421–427. doi: 10.1016/S1438-4221(00)80056-9. [DOI] [PubMed] [Google Scholar]
- [20].Tournier JN, Rossi PS, Quesnel-Hellmann A, Baldari CT. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009;30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [21].Moayeri M, Martinez NW, Wiggins J, Young HA, Leppla SH. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 2004;72:4439–4447. doi: 10.1128/IAI.72.8.4439-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS. ONE. 2008;3:e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ezzell JW, Ivins BE, Leppla SH. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 1984;45:761–767. doi: 10.1128/iai.45.3.761-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beall FA, Taylor MJ, Thorne CB. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J. Bacteriol. 1962;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cui X, Li Y, Li X, Haley M, Moayeri M, Fitz Y, Leppla SH, Eichacker PQ. Sublethal doses of Bacillus anthracis lethal toxin inhibit inflammation with lipopolysaccharide and Escherichia coli challenge but have opposite effects on survival. J. Infect. Dis. 2006;193:829–840. doi: 10.1086/500468. [DOI] [PubMed] [Google Scholar]
- [26].Sherer K, Li Y, Cui X, Li X, Subramanian M, Laird MW, Moayeri M, Leppla SH, Fitz Y, Su J, Eichacker PQ. Fluid support worsens outcome and negates the benefit of protective antigen-directed monoclonal antibody in a lethal toxin-infused rat Bacillus anthracis shock model. Crit. Care Med. 2007;35:1560–1567. doi: 10.1097/01.CCM.0000266535.95770.A2. [DOI] [PubMed] [Google Scholar]
- [27].Li Y, Cui X, Su J, Haley M, Macarthur H, Sherer K, Moayeri M, Leppla SH, Fitz Y, Eichacker PQ. Norepinephrine increases blood pressure but not survival with anthrax lethal toxin in rats. Crit. Care Med. 2009;37:1348–1354. doi: 10.1097/CCM.0b013e31819cee38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuo SR, Willingham MC, Bour SH, Andreas EA, Park SK, Jackson C, Duesbery NS, Leppla SH, Tang WJ, Frankel AE. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb. Pathog. 2008;44:467–472. doi: 10.1016/j.micpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- [29].Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS. ONE. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Watson LE, Mock J, Lal H, Lu G, Bourdeau RW, Tang WJ, Leppla SH, Dostal DE, Frankel AE. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front. Biosci. 2007;12:4670–4675. doi: 10.2741/2416. [DOI] [PubMed] [Google Scholar]
- [31].Hicks CW, Li Y, Okugawa S, Solomon SB, Moayeri M, Leppla SH, Mohanty A, Subramanian GM, Mignone TS, Fitz Y, Cui X, Eichacker PQ. Anthrax edema toxin has cAMP-mediated stimulatory effects and high-dose lethal toxin has depressant effects in an isolated perfused rat heart model. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1108–H1118. doi: 10.1152/ajpheart.01128.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS. Pathog. 2009;4:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McAllister RD, Singh Y, Du Bois WD, Potter M, Boehm T, Meeker ND, Fillmore PD, Anderson LM, Poynter ME, Teuscher C. Susceptibility to anthrax lethal toxin is controlled by three linked quantitative trait loci. Am. J. Pathol. 2003;163:1735–1741. doi: 10.1016/S0002-9440(10)63532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- [35].Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- [37].Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, Reed JC. Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3935–3940. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, Matsuzawa S, Terskikh AV, Faustin B, Reed JC. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- [39].Muehlbauer SM, Evering TH, Bonuccelli G, Squires RC, Ashton AW, Porcelli SA, Lisanti MP, Brojatsch J. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007;6:758–766. doi: 10.4161/cc.6.6.3991. [DOI] [PubMed] [Google Scholar]
- [40].Reig N, Jiang A, Couture R, Sutterwala FS, Ogura Y, Flavell RA, Mellman I, van der Goot FG. Maturation modulates caspase-1-independent responses of dendritic cells to anthrax lethal toxin. Cell. Microbiol. 2008;10:1190–1207. doi: 10.1111/j.1462-5822.2008.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Newman ZL, Crown D, Leppla SH, Moayeri M. Anthrax lethal toxin activates the inflammasome in sensitive rat macrophages. Biochem. Biophys. Res. Commun. 2010;398:785–789. doi: 10.1016/j.bbrc.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Muehlbauer SM, Lima H, Jr., Goldman DL, Jacobson LS, Rivera J, Goldberg MF, Palladino MA, Casadevall A, Brojatsch J. Proteasome inhibitors prevent caspase-1-mediated disease in rodents challenged with anthrax lethal toxin. Am. J. Pathol. 2010;177:735–743. doi: 10.2353/ajpath.2010.090828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wickliffe KE, Leppla SH, Moayeri M. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 2008;10:332–343. doi: 10.1111/j.1462-5822.2007.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nour AM, Yeung YG, Santambrogio L, Boyden ED, Stanley ER, Brojatsch J. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect. Immun. 2009;77:1262–1271. doi: 10.1128/IAI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Levin TC, Wickliffe KE, Leppla SH, Moayeri M. Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell. Microbiol. 2008;10:2434–2446. doi: 10.1111/j.1462-5822.2008.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect. Immun. 2009;77:4327–4336. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Newman ZL, Sirianni N, Mawhinney C, Lee MS, Leppla SH, Moayeri M, Johansen LM. Auranofin protects against anthrax lethal toxin-induced Nlrp1b-inflammasome activation. Antimicrob. Agents Chemother. 2010;55:1028–1035. doi: 10.1128/AAC.00772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liao KC, Mogridge J. Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin. Infect. Immun. 2009;77:4455–4462. doi: 10.1128/IAI.00276-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hanna PC, Kruskal BA, Ezekowitz RA, Bloom BR, Collier RJ. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol. Med. 1994;1:7–18. [PMC free article] [PubMed] [Google Scholar]
- [50].Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- [51].Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thomas J, Epshtein Y, Chopra A, Ordog B, Ghassemi M, Christman JW, Nattel S, Cook JL, Levitan I. Anthrax lethal factor activates K+ channels to induce IL-1B, secretion in macrophages. J. Immunol. 2011;186:5236–5243. doi: 10.4049/jimmunol.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Squires RC, Muehlbauer SM, Brojatsch J. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 2007;282:34260–34267. doi: 10.1074/jbc.M705687200. [DOI] [PubMed] [Google Scholar]
- [54].Alileche A, Squires RC, Muehlbauer SM, Lisanti MP, Brojatsch J. Mitochondrial impairment is a critical event in anthrax lethal toxin-induced cytolysis of murine macrophages. Cell Cycle. 2006;5:100–106. doi: 10.4161/cc.5.1.2283. [DOI] [PubMed] [Google Scholar]
- [55].Varshavsky A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 2011;20:1298–1345. doi: 10.1002/pro.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wickliffe KE, Leppla SH, Moayeri M. Killing of macrophages by anthrax lethal toxin: involvement of the N-end rule pathway. Cell. Microbiol. 2008;10:1352–1362. doi: 10.1111/j.1462-5822.2008.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ha SD, Ng D, Lamothe J, Valvano MA, Han J, Kim SO. Mitochondrial proteins Bnip3 and Bnip3L are involved in anthrax lethal toxin-induced macrophage cell death. J. Biol. Chem. 2007;282:26275–26283. doi: 10.1074/jbc.M703668200. [DOI] [PubMed] [Google Scholar]
- [58].Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee S, Wang Y, Kim SO, Han J. AMPD3 is involved in anthrax LeTx-induced macrophage cell death. Protein Cell. 2011;2:564–572. doi: 10.1007/s13238-011-1078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chapelsky S, Batty S, Frost M, Mogridge J. Inhibition of anthrax lethal toxin-induced cytolysis of RAW264.7 cells by celastrol. PLoS. ONE. 2008;3:e1421. doi: 10.1371/journal.pone.0001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Averette KM, Pratt MR, Yang Y, Bassilian S, Whitelegge JP, Loo JA, Muir TW, Bradley KA. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS. ONE. 2009;4:e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ha SD, Ham B, Mogridge J, Saftig P, Lin S, Kim SO. Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. J. Biol. Chem. 2009;285:2120–2129. doi: 10.1074/jbc.M109.065813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase Inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- [64].Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011 doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Moayeri M, Wickliffe KE, Wiggins JF, Leppla SH. Oxidized ATP protection against anthrax lethal toxin. Infect. Immun. 2006;74:3707–3714. doi: 10.1128/IAI.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ngai S, Batty S, Liao KC, Mogridge J. An anthrax lethal factor mutant that is defective at causing pyroptosis retains proapoptotic activity. FEBS J. 2010;277:119–127. doi: 10.1111/j.1742-4658.2009.07458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, Levine SM, Bradley KA. Cutting edge: Resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J. Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moayeri M, Webster JI, Wiggins JF, Leppla SH, Sternberg EM. Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect. Immun. 2005;73:4238–4244. doi: 10.1128/IAI.73.7.4238-4244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yazdi AS, Drexler SK, Tschopp J. The role of the inflammasome in nonmyeloid cells. J. Clin. Immunol. 2010;30:623–627. doi: 10.1007/s10875-010-9437-y. [DOI] [PubMed] [Google Scholar]
- [71].Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- [72].Watters JW, Dewar K, Lehoczky J, Boyartchuk V, Dietrich WF. Kif1C, a kinesin-like motor protein, mediates mouse macrophage resistance to anthrax lethal factor. Curr. Biol. 2001;11:1503–1511. doi: 10.1016/s0960-9822(01)00476-6. [DOI] [PubMed] [Google Scholar]
- [73].Ross JM. The pathogenesis of anthrax following administration of spores by the respiratory route. J. Pathol. Bacteriol. 1957;73:485–494. [Google Scholar]
- [74].Guidi-Rontani C, Levy M, Ohayon H, Mock M. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 2001;42:931–938. doi: 10.1046/j.1365-2958.2001.02695.x. [DOI] [PubMed] [Google Scholar]
- [75].Cote CK, DiMezzo TL, Banks DJ, France B, Bradley KA, Welkos SL. Early interactions between fully virulent Bacillus anthracis and macrophages that influence the balance between spore clearance and development of a lethal infection. Microbes. Infect. 2008;10:613–619. doi: 10.1016/j.micinf.2008.02.006. [DOI] [PubMed] [Google Scholar]
- [76].Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lincoln RE, Walker JS, Klein F, Rosenwald AJ, Jones WI., Jr. Value of field data for extrapolation in anthrax. Fed. Proc. 1967;26:1558–1562. [PubMed] [Google Scholar]
- [78].Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, Liu S, Sastalla I, Leppla SH. Inflammasome sensor Nlrp1b-dependent resistance to anthrax Is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Klein F, Haines BW, Mahlandt BG, Dearmon IA, Jr., Lincoln RE. Dual nature of resistance mechanisms as revealed by studies of anthrax septicemia. J. Bacteriol. 1963;85:1032–1038. doi: 10.1128/jb.85.5.1032-1038.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]