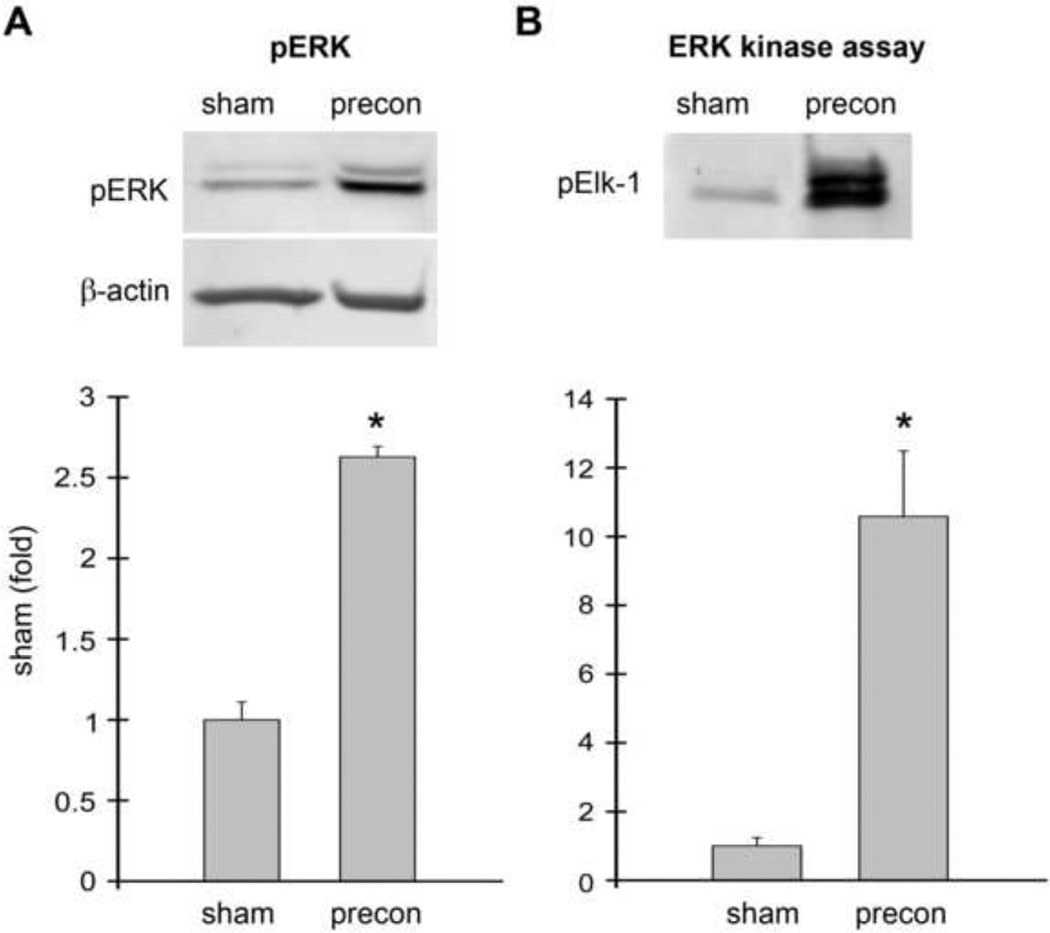
Fig. 2. ERK1/2 phosphorylation and activity was increased in rats receiving ischemic preconditioning alone without stroke.
Ischemic preconditioning was performed 1 h prior to permanent MCAo by transient MCAo with a micro clip for 15 min. Rat brains were harvested 1 h after preconditioning; no test ischemia was induced. The brain region corresponding to ischemic penumbra (region I defined in Fig.1) was dissected for analysis. Rat brains were processed for Western blot and ERK1/2 kinase assay. A. Western blot showing RIPC increased p-ERK1/2 levels (top). Two protein bands were observed. The top band is pERK1 (44 kDa), and the lower band is pERK2 (42kDa). The bar graph shows the average optical densities of the protein bands (bottom). B. RIPC also increased ERK1/2 activity as assessed by in vitro kinase assay compared to sham. Activated protein kinase phosphorylates its substrates, thus levels of a phophorylated protein substrate can be used as a marker for protein kinase activity. In this experiment, ERK kinase activity was determined by the amount of Elk-1 phosphorylated by ERK1/2; p-Elk-1 was detected by Western blot. * vs sham, P<0.05. N=3–6/group.