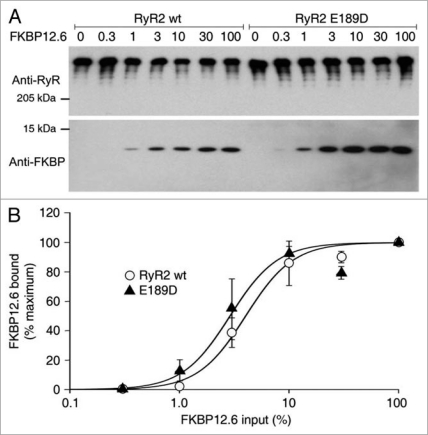
Figure 3.
E189D does not alter the affinity of binding of FKBP12.6 to RyR2. (A) Western blots showing the interaction between RyR2 wt or E189D with various amounts of FKBP12.6 (values indicate % of maximal input). The RyR2-FKBP12.6 complex was co-immunoprecipitated using an anti-RyR antibody followed by immunoblotting with anti-RyR (upper part) and anti-FKBP12/12.6 (lower part) antibodies. Results shown are representative of three separate experiments. (B) Ratio of FKBP12.6 to RyR2, calculated using densitometry of the protein bands, normalized to maximal binding showed that there are no significant differences in the level of protein at any given FKBP12.6 concentration (p = 0.1–0.4).