Fig. 2.
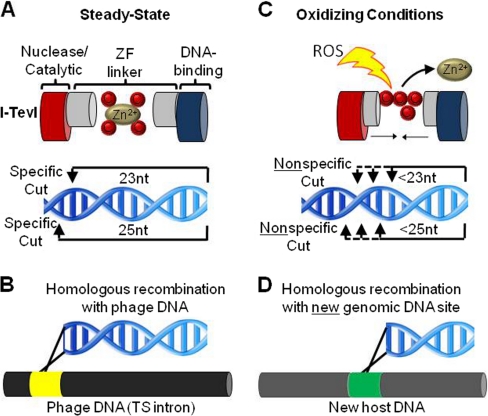
Crosstalk between metal binding and redox regulation. A, The intron endonuclease I-TevI has two domains, a DNA-binding domain and a catalytic nuclease domain, separated by a linker region that uses a zinc finger (ZF) to stabilize the extended structure. Under steady-state conditions the linker is fully extended and the nuclease cleaves 23 and 25 nucleotides from the DNA-binding site. B, This allows maintenance of the endonuclease in an intron of the thymidylate synthase gene (TS intron) of the bacteriophage T4. C, Hydrogen peroxide-induced oxidation disrupts the ZF, shortening the linker between the DNA-binding domain and the nuclease domain leading to shorter, nonspecific DNA cleavage (3). D, Although I-TevI typically recombines within an intronless TS gene, the nonspecifically cleaved DNA sequences which result due to oxidation of I-TevI can homologously recombine at a new genomic site or host.