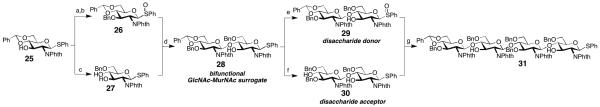
Scheme 5.
Reagents and conditions: a) BnBr (1.1 equiv.), NaH (1.2 equiv.), Bu4NI (0.1 equiv.), DMF, rt, 70%; b) mCPBA (1 equiv.), CH2Cl2, −78 °C to rt, 88%; c) HSiEt3 (5 equiv.), TFA (5 equiv.), CH2Cl2, 0 °C, 93%; d) 27 (1.5 equiv.), ADMB (5 equiv.), DTBMP (5 equiv.), Tf2O (1 equiv.), CH2Cl2, MS 4Å, 58 %; e) mCPBA (1.1 equiv.), CH2Cl2, 77%; f) HSiEt3 (12 equiv.), BF3·OEt2 (2 equiv.), CH2Cl2, 70%; g) 29 (1.25 equiv.), ADMB (6.25 equiv.), DTBMP (3.75 equiv.), Tf2O (1.25 equiv.), CH2Cl2, MS 4Å, 55%. Abbreviations: ADMB = 4-allyl-1,2-dimethoxybenzene, DTBMP = 2,6-di-tert-butyl-4-methylpyridine.