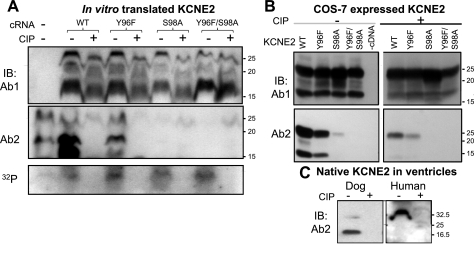
Fig. 6.
Detection of KCNE2 by Ab2 requires phosphorylation of serine at position 98 (S98). A: KCNE2 wild-type (WT) and 3 mutants (Y96F, S98A, and Y96F/S98A) are in vitro translated in the presence of γ-32P-ATP to label proteins phosphorylated during translation. The translation products are divided into 2 aliquots, 1 treated with calf intestinal phosphatase (CIP) and the other processed in the same manner without CIP. The aliquots are fractionated by 2 separate SDS-PAGE; 1 is used to probe with Ab1 (top) and the other with Ab2 (middle). Remaining 32P-radioactivity in the gel (in the 10–30 kDa range) is revealed by phosphoimager (bottom). The farthest left lane is no cRNA negative control. B: WCL from COS-7 cells expressing KCNE2 WT or mutants (listed on top), without or with CIP dephosphorylation (− and +, respectively), probed with Ab1 (top) and Ab2 (bottom). C: WTL from dog and human ventricles are divided into 2 aliquots, 1 treated with CIP and the other processed in the same manner without CIP. The 2 aliquots are fractionated side by side and probed with Ab2. CB stain confirms even loading (not shown). In all 3 panels, size marker positions are marked on the right.