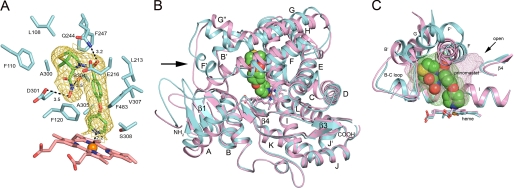
FIGURE 3.
A, amino acid side chains (cyan carbons) contacting prinomastat (green carbons) are depicted along with the heme prosthetic group (pink carbons) as stick figures. The gold mesh depicts a 2|Fo| − |Fc| σA-weighted prinomastat omit map contoured at 1σ around the prinomastat molecule. Dashed lines identify hydrogen bonds and the coordination of the pyridyl moiety with the heme iron (orange sphere) with the interatomic distances shown. Amino acid residues are identified by single letter amino acid codes and residue number. Nitrogen, oxygen, sulfur and iron atoms are colored blue, red, yellow, and orange, respectively. B, ribbon diagram showing the superposition of the structure of the prinomastat complex 3QM4 with the substrate-free structure 2F9Q (carbons colored light magenta). Helices are designated by letters and sheets by numbers. The arrow indicates helix F′ seen in the structure of the prinomastat complex. The surrounding region exhibits significant structure differences. C, comparison of the shapes of the active site cavities is depicted by a mesh surface colored green for the prinomastat complex and light magenta for 2F9Q structure. Cavity surfaces were generated by VOIDOO using a 1.4-Å probe. Water molecules were used to terminate the exterior opening of the solvent channel seen for the 2F9Q structure (arrow).