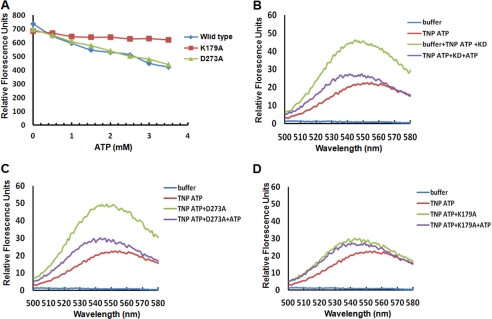
FIGURE 2.
Analysis of ATP binding by fluorescence spectroscopy. A, intrinsic fluorescence of the wild type kinase domain and the indicated mutants upon ATP binding by measuring emission at 335 nm. B, fluorescence spectra of TNP-ATP bound to wild-type kinase domain. The fluorescence excitation and emission of TNP-ATP was at 403 nm and 547 nm, respectively. After recording, excess ATP was added to displace the bound TNP-ATP from the enzyme, which resulted in decreased fluorescence. C, fluorescence spectra of TNP-ATP bound to the D273A mutant of the kinase domain as in B. D, fluorescence spectra of K179A mutant bound to TNP-ATP. TNP-ATP and subsequent addition of excess ATP indicate that Lys179 is crucial for ATP binding.