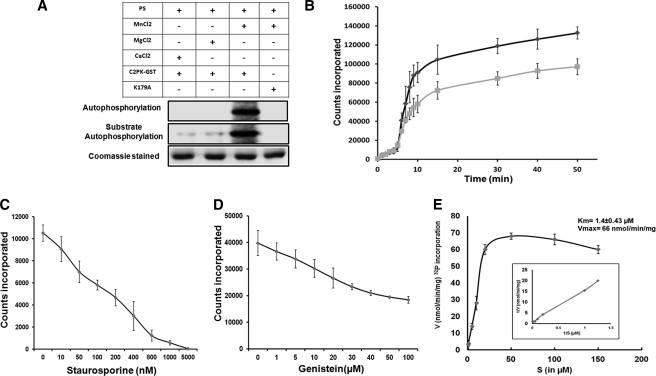
FIGURE 3.
Phosphorylation activity of kinase domain of EhC2PK. A, effect of indicated lipids and divalent cations on autophosphorylation and substrate phosphorylation activities of GST-tagged full-length EhC2PK. B, time kinetics of phosphorylation activity of kinase domain (black line) and full-length EhC2PK (gray line). C, phosphorylation activity of the kinase domain in the presence of varying concentrations of staurosporine. D, phosphorylation activity of the kinase domain in the presence of varying concentrations of genistein. E, Michelis-Menten parameters for wild-type kinase domain of EhC2PK. All kinase reactions were carried out using recombinant proteins as indicated. Reaction mixture contained 10 μCi per reaction of [32P-γ]ATP.