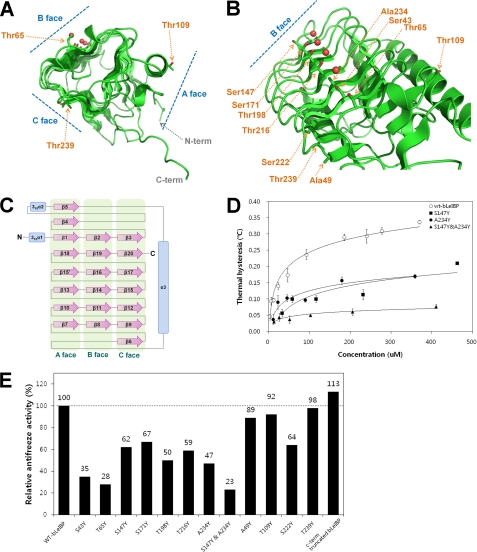
FIGURE 4.
Summary of mutation effects on LeIBP. A, the location of each mutation on the trigonal cylinder structure is shown in the stick model. Ordered water molecules (red spheres) are presented on the B face beside the aligned Thr/Ser/Ala residues. B, amino acid side chains mutated in these studies are labeled. The mutant proteins were purified to near homogeneity and were compared with wild-type non-glycosylated LeIBP for their antifreeze activities. C, topology diagram of LeIBP structure. α-Helices and β-strands are shown as cylinders and arrows, respectively. D, the TH activities of wild-type and mutant non-glycosylated LeIBP are plotted as a function of concentration. E, comparisons of the antifreeze activities of the wild-type non-glycosylated LeIBP and the mutants at 370 μm concentration.