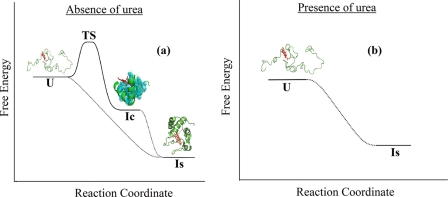
FIGURE 8.
A schematic diagram showing the sodium perchlorate induced formation of IC and IS in aqueous buffer (a) and in the presence (b) of 3 m urea. The unfolded extended conformer (U) does not have any secondary structure although the cofactor heme is bound to the protein. U has been found to be in rapid equilibrium with a compact conformer (IC) and the time constant of their interconversion is 50 μs. The addition of sodium perchlorate shifts the equilibrium toward IC, which is accompanied by a decrease in rH and an increase in F. The hydrophobic residues, which may be involved in the formation of IC, are shown by space-filling spheres. The cofactor heme, because it is hydrophobic, may also contribute toward the formation of IC. IC has been shown to contain partial secondary structure (presumably at the N and C-terminal helix regions). Sodium perchlorate induced formation of IC is a sharp cooperative transition with a defined transition state. The formation of the secondary structure (IS) takes place gradually over an extended range of sodium perchlorate concentration. This is an extended homopolymer-like transition and shown using dotted lines. The formation of the native protein is slow and is not shown. No rapid equilibrium exists between the extended (U) and any IC-like state in the presence of 3 m urea. A small extent of chain contraction (observed by the decrease in rH) and an increase in the secondary structure formation occur slowly and simultaneously in the presence of urea. The structure of IC (or IS) in the presence of urea is not defined, although these states are more compact than U and more extended than N (or IC observed without urea).