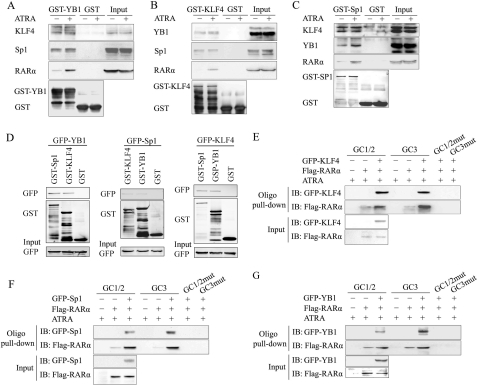
FIGURE 5.
ATRA promoted the binding of RARα to the Klf4 promoter in a KLF4-Sp1-YB1-dependent manner. A, the purified recombinant GST and GST-YB1 proteins on the glutathione beads were incubated with total cell lysates of VSMCs treated with 10 μm ATRA for 1 h, followed by extensive washing. Proteins on the beads were subjected to Western blot with anti-KLF4, anti-Sp1, or anti-RARα antibodies. B, GST pulldown assay of recombinant GST-KLF4 or GST with lysates from VSMCs treated with ATRA for 1 h. Proteins on the beads were subjected to Western blot with anti-YB1, anti-Sp1, and anti-RARα antibodies. C, GST pulldown assay of recombinant GST-Sp1 or GST with lysates from VSMCs treated with ATRA for 1 h. Proteins on the beads were subjected to Western blot with anti-KLF4, anti-YB1, or anti-RARα antibodies. D, CHO-K1 cells were transfected for 24 h to express GFP-YB1 (left), GFP-Sp1 (middle), or GFP-KLF4 (right) protein and then treated with 10 μm of ATRA for 1 h. The whole cell lysates of CHO-K1 cells were used to perform GST pulldown assay. The recombinant GST, GST-KLF4, GST-Sp1, and GST-YB1 proteins on the glutathione beads were incubated with the cell lysates, respectively, overnight at 4 °C, followed by extensive washing. Proteins on the beads were eluted and detected by Western blot with anti-GFP antibody. E–G, CHO-K1 cells were co-transfected to express indicated factors, with a FLAG tag attached to RARα and with a GFP tag attached to KLF4 (E), Sp1 (F), or YB1 (G). The cell lysates were subjected to an oligonucleotide pulldown assay with biotinylated double-stranded oligonucleotides containing wild-type GC1 and GC2 box sequences (GC1/2), mutated GC1 and GC2 (GC1/2mut), wild-type GC3 (GC3), and mutated GC3 (GC3mut) as probes. The precipitates were analyzed by Western blot with antibodies against the FLAG tag or GFP tag.