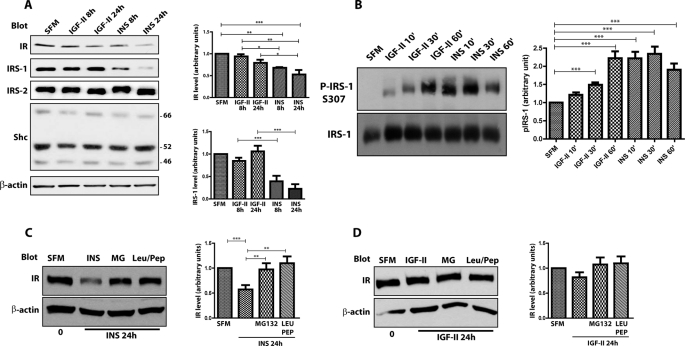
FIGURE 3.
Insulin and IGF-II differ in their ability to regulate IR-A and IRS-1 stability. A, R−/IR-A cells were serum starved for 24 h and then stimulated with 50 ng/ml of insulin (INS) or IGF-II for the indicated time points. Proteins levels were determined by immunoblot analysis with specific polyclonal antibodies, as described under “Experimental Procedures.” B, serum-starved R−/IR-A cells were stimulated at the different time points with insulin and IGF-II (50 ng/ml). Serine phosphorylation of IRS-1 was determined by immunoblot using phospho-specific antibodies for Ser307. Total IRS-1 was assessed using anti-IRS-1 polyclonal antibodies. Densitometric analysis is expressed as arbitrary units. B, serine phosphorylation of IRS-1 was assessed by immunoblot using phospho-specific antibodies for serine 307. Blots are representative of three independent experiments. C and D, to assess the stability of the IR-A in the presence of specific inhibitors for either the proteasomal or lysosomal pathway, serum-starved R−/IR-A cells were stimulated for 24 h with 50 ng/ml of insulin (INS) (C) or IGF-II (D) alone or supplemented with 20 μm MG132 (MG) or 100 μm leupeptin/pepstatin (Leu/Pep). IR levels were assessed by immunoblot. The total amount of protein loaded on the gel was monitored using anti-β-actin polyclonal antibodies (A–E). Quantification was performed by densitometry using NIH ImageJ software. The data are presented as mean ± S.D. Statistical significance was determined using two-way ANOVA with Bonferroni's multiple-comparison test. ***, p < 0.001; **, p < 0.01; *, p < 0.05.