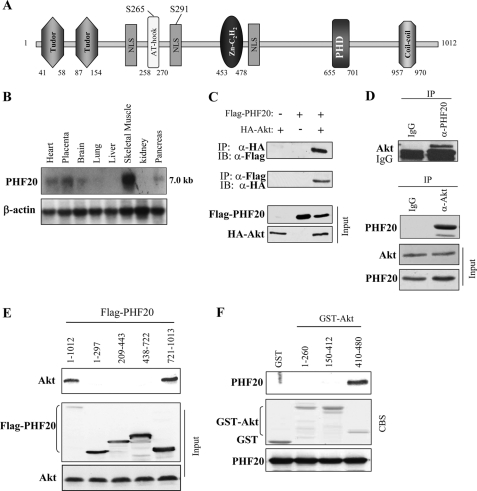
FIGURE 1.
PHF20 interacts with Akt. A, schematic structure of PHF20. PHF20 contains two Tudor, an AT-hook, a C2H2 zinc finger, and a PHD finger domain, three nuclear localization signals (NLS), as well as two putative Akt phosphorylation consensus sites. B, Northern blot analysis shows the expression of PHF20 in multiple human tissues. C, PHF20 binds to Akt. HEK293 cells were co-transfected with FLAG-PHF20 and HA-Akt. After incubation for 48 h, cells were lysed, immunoprecipitated (IP), and immunoblotted (IB) with the indicated antibodies. D, interaction of PHF20 with Akt at endogenous protein levels. Immunoprecipitation and immunoblotting analysis were performed with indicated antibodies in HCT116 cells. E, C-terminal region of PHF20 binds to Akt. MCF7 cells were transfected with the different truncation mutants of FLAG-PHF20, lysed, and immunoprecipitated with FLAG antibody. The immunoprecipitates were immunoblotted with Akt antibody (top). Middle and bottom panels show expression of FLAG-PHF20 and Akt, respectively. F, C-terminal domain of Akt interacts with PHF20. GST pulldown assay was performed by incubation of GST, GST-fused Akt proteins, with in vitro translated PHF20. The pulldown products were immunoblotted with PHF20 antibody (top). The middle panel is Coomassie Blue staining (CBS) and the bottom panel is immunoblotting of the in vitro translated products with PHF20 antibody.