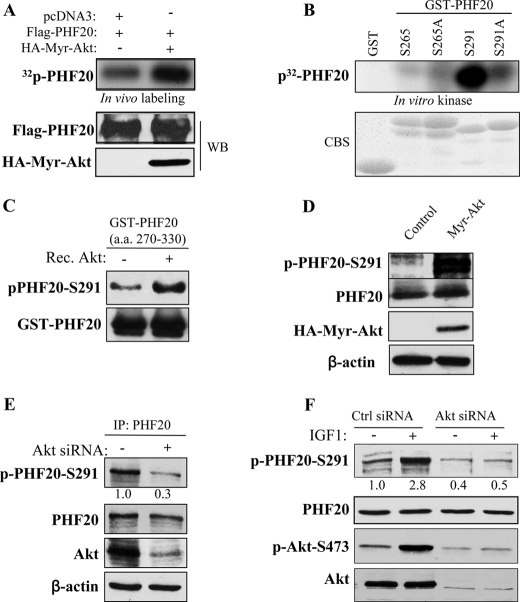
FIGURE 2.
Akt phosphorylates PHF20 in vitro and in vivo. A, PHF20 was phosphorylated by Akt in vivo. In vivo labeling was performed as described under “Experimental Procedures.” Briefly, HEK293 cells were co-transfected with FLAG-PHF20 and constitutively active HA-Myr-Akt or pcDNA3 and labeled with [32P]orthophosphate (0.5 mCi/ml) for 4 h. Cell lysates were immunoprecipitated with FLAG antibody and separated by SDS-PAGE and transferred to membrane. The phosphorylated PHF20 band was examined by autoradiography (top). Middle and bottom panels show the expression of transfected plasmids. B and C, Akt phosphorylates PHF20 in vitro. In vitro kinase assay was performed by incubation of recombinant active Akt with GST-fused PHF20 amino acids 200–280 (Ser265) and 270–330 (Ser291) and their mutant forms. The relative amount of incorporated radioactivity was determined by autoradiography (B) and phospho-PHF20-Ser291 antibody (C). D, Akt phosphorylates PHF20-Ser291 in vivo. HCT116 cells were transfected with constitutively active HA-Myr-Akt or pcDNA3. After incubation of 48 h, cell lysates were immunoblotted with indicated antibodies. E and F, knockdown of Akt reduces phospho-PHF20-Ser291. MDA-MB-468 (E) and MCF7 (F) cells were transfected with Akt siRNA or control siRNA. After 72 h, cell lysates were immunoblotted with indicated antibodies (E). F shows that Akt siRNA or control siRNA-treated MCF7 cells were serum-starved for 12 h, stimulated with IGF1 for 2 h and then immunoblotted with indicated antibodies. WB, Western blot; CBS, Coomassie Blue staining; IP, immunoprecipitation.